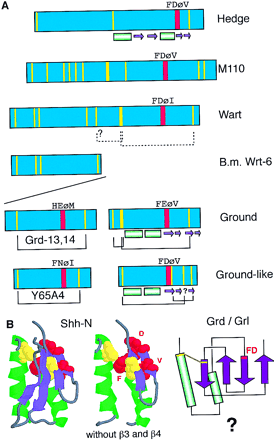
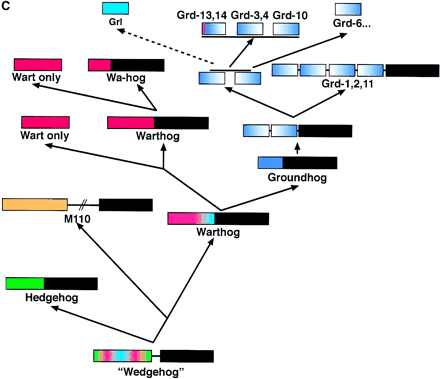
Hypothesis for the structure and evolution of the hh, wrt, grd, and grl genes. The green bars denote α-helixes, and the purple arrows β-strands in A and B. (A) Schematic representation of the different conserved domains and the amino-terminal region of M110. The yellow lines indicate conserved cysteine residues. The red box indicates the conserved FDφV motifs found in variations in all sequences. The brackets indicate putative cysteine disulfide bonds. Underneath the several domains are schematic representations of the secondary structure (Hedge) or secondary structure predictions (Grd, Grl). (B) The central part of the Shh-H structure viewed with RasMol; (right) β-strand 3 and 4 were removed. The FDφV motif is represented in red, the cysteine residues in yellow. The scheme at right is a topologically allowed scheme of how the Ground and Ground-like domain could be arranged. (C) Evolution of the hog gene families from a common ancestralwedgehog gene. Genes are represented schematically; the regions between the domains not drawn to scale. The black box denotes the Hog region; the other colors the different amino-terminal domains, i.e., Hedge, Wart, Ground, and Ground-like.











