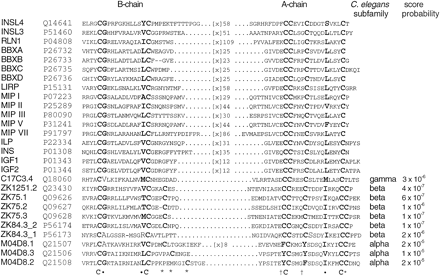
Alignment of C. elegans ILPs with representatives of all currently identified insulin-related families. (INSL4) Human early placenta ILP (EPIL); (INSL3) human Leydig ILP (LEY I L); (RLN1) human prorelaxin H1; (BBXA, BBXB, BBXC, BBXD) B. mori bombyxin (BBX) A9, B1, C2, and D1; (LIRP) locust insulin-related peptide. (MIP I, MIP II, MIP III, MIP V, MIP VII) Molluscan insulin-related peptides; (ILP) Amphioxus ILP; (INS) human insulin; (IGF1, IGF2) human insulin-like growth factor I and II (IGF II). C. elegans insulin-like peptides: (C17C3.4) γ type; (ZK1251.2, ZK75.1, ZK75.2, ZK75.3, ZK84.3_1, ZK84.3_2: β-type; (M04D8.1, M04D8.2, M04D8.3) α type. (*) Positions of cysteines involved in the additional disulfide bond between B and A chains of α and β types. Daggers indicate positions of Phe or Tyr substituting the disulfide bond within A chain of α type; (C) conserved cysteines; (•) other conserved positions. SWISS-PROT/TREMBL accession nos. are shown. The length of the nonconserved peptide linking B and A chains is indicated.











