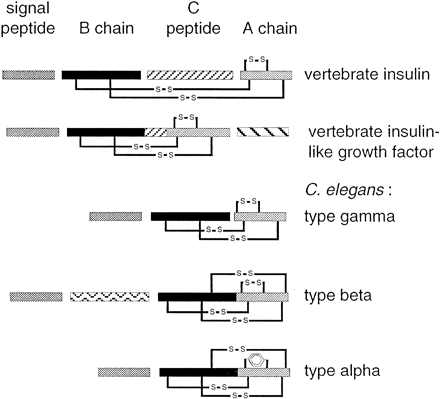
Schematic representation of vertebrate insulin, IGF, and C. elegans insulin-like predicted proteins. Domains that are cleaved during maturation of the propeptide are separated by spaces. Disulfide bonds are indicated. C. elegans insulin-like proteins are characterized by the absence of C-peptide. Type β and type α have no potential cleavage site between B and A chains; type β and type α contain an additional disulfide bond between B and A chains. In type-α proteins, the disulfide bond within the A chain is substituted by hydrophobic interaction between Phe and/or Tyr residues.











