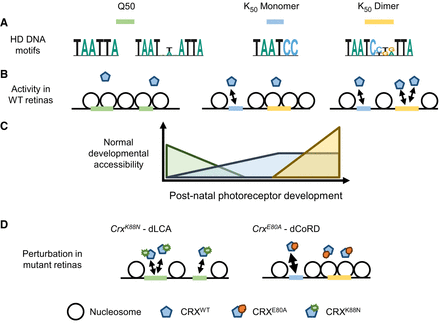
Differential HD motif usage in photoreceptor development and diseases. (A) Q50 and K50 paired-class HDs prefer different monomeric and dimeric DNA motifs. (B,C) In postnatal photoreceptor precursors, regulatory elements with Q50 HD motifs are gradually closed (silenced) owing to a lack of interacting TFs. High-affinity monomeric K50 HD motifs respond to low concentration of CRX, drive chromatin remodeling in early-stage development, and plateau in their regulatory output at a later stage. Dimeric K50 HD motifs of individually low-affinity half-sites respond at high CRX concentration and are associated with chromatin remodeling and gene expression regulation at photoreceptor terminal differentiation. (D) In CrxK88N mutant retinas, CRXK88N’s ectopic binding impedes the silencing of regulatory elements with Q50 HD motifs. In CrxE80A mutant retinas, CRXE80A’s enhanced interactions with monomeric K50 HD motifs and defective cooperative binding at dimeric K50 HD motifs lead to accelerated chromatin remodeling in early-stage development but defective remodeling at terminal differentiation stage. (dLCA) Dominant Leber congenital amaurosis, (dCoRD) dominant cone-rod dystrophy.











