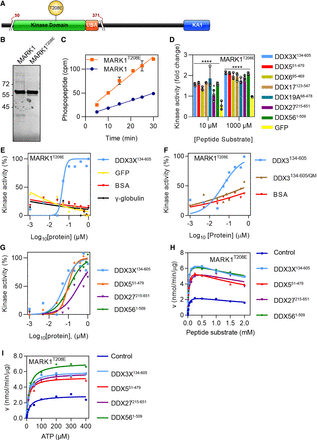
MARK1 activity is stimulated by DDX proteins in vitro. (A) Schematic of MARK1 with the boundaries indicated in red for the truncated recombinant protein used (aa 50–371). (UBA) Ubiquitin association domain, (KA1) kinase-associated domain 1, and (T208E) phosphomimetic mutation for activated MARK1. Cartoon generated using IBS v1.0.3 (Liu et al. 2015). (B) SDS-PAGE gel stained with Coomassie blue of MARK1 expressed in and purified from E. coli. (C) In vitro kinase assay linearity of MARK1 and MARK1T208E. (D) In vitro kinase assay with MARK1T208E with the addition of different DDX core domain proteins both at low (10 µM) and high (1000 µM) peptide substrate. (****) P > 0.0001. (E) In vitro kinase assay using MARK1T208E and increasing amounts of either DDX3X132–605 or different negative control proteins for EC50 determination. (F) In vitro kinase assay using MARK1T208E and increasing amounts of either DDX3X132–605, DDX3 QM, or BSA as control proteins. (G) In vitro kinase assay using MARK1T208E and increasing amounts of DDX core domain proteins for EC50 determination. (H) In vitro kinase assay using MARK1T208E with the addition of DDX core domain proteins and increasing concentrations of peptide substrate at saturating ATP concentration. (I) In vitro kinase assay using MARK1T208E with the addition of DDX core domain proteins and increasing concentrations of ATP at saturating peptide concentration.











