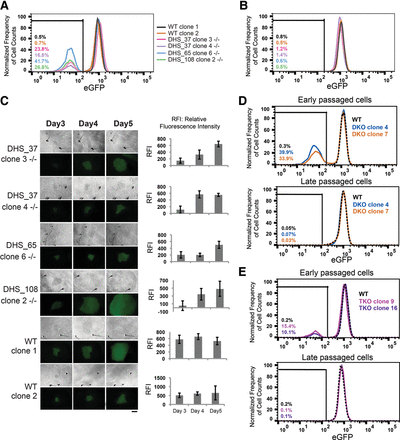
Characterization of Temp enhancers. (A–C) Biallelic clones with single deletions at DHS_37, DHS_65, and DHS_108 loci were generated by cotransfection of a pair of pX330 plasmids expressing sgRNAs flanking each locus. Each clone was cultured for 2 wk before their POU5F1 expression levels were assessed using FACS analysis of eGFP in early passages (A) and late passages (B). (A) In early passages, biallelic mutant clones showed heterogeneous eGFP expression levels. (B) eGFP− cells isolated from (A) restored eGFP level. (C) Colony image tracing experiments showed that mutant clones gradually regain eGFP expression in culture over time. Bar graph represents average relative fluorescence intensity (RFI) for each colony quantified by ImageJ. Scale bar, 200 μm. (D) DHS_37 and DHS_108 (−/−, −/−) double knockout (DKO) clones were generated by two rounds of deletion of each region by CRISPR/Cas9 and confirmed by Sanger sequencing. DKO cells exhibit transient loss of eGFP expression that can be recovered after long-term culture. (E) DHS_37, DHS_65, and DHS_108 (−/−, −/−, −/−) triple knockout (TKO) clones were generated by three rounds of deletion of regions by CRISPR/Cas9 and confirmed by Sanger sequencing. TKO cells exhibit transient loss of eGFP expression that can be recovered after long-term culture.











