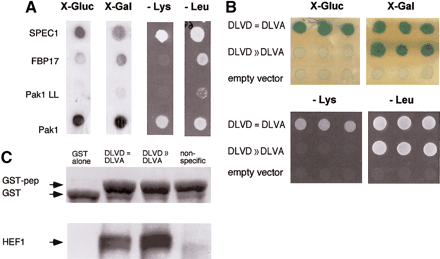
Targeting of interacting proteins or peptides to small sequence motifs. (A) Differential interaction of Cdc42 effectors as detected by the Dual Bait procedure. Two Cdc42-interacting proteins, FBP17 (Fuchs et al. 2001) and SPEC1 (Pirone et al. 2000) were isolated by a yeast two-hybrid screen with LexA-Cdc42-L28. In this screen, 107diploids arising from an interaction mating (Finley and Brent 1994)-based screen yielded 25 positive colonies showing interactions with LexA-Cdc42 L28. Counterscreening against cI fused to activated Cdc42 lacking the insert region (Cdc42 L28-Δ8) revealed two clones, both containing the same cDNA, showing differential binding. All yeast shown contain both baits, and AD-fusions as indicated at left. Beside AD-fused FBP17 (specific for Cdc42-L28) and SPEC1 (bound both forms equivalently), AD-Pak1, which binds both forms of Cdc42, and AD-Pak1 LL, which cannot bind either form of Cdc42 (Sells et al. 1997), are included as controls. Results with growth and colorimetric reporters for Cdc42-L28-Δ8 [gusA (X-Gluc) and LYS2, first and third lines] and LexA-Cdc42-L28 [lacZ (X-Gal) andLEU2, second and fourth lines], are shown. (B) Selection of peptides specifically targeting HEF1-DLVD. A LexA-HEF1(DLVD) bait was used to identify interacting peptide aptamers, in collaboration with R. Finley. Selected peptides were rescreened in parallel against LexA-HEF1(DVLD) or cI-HEF1(DLVA), differing from the original bait by only a D363A substitution. Shown are peptides that interact with both DLVD and DLVA variants (DLVD = DLVA; activates all four reporters; representative sequence HHASTPRRESPGIMSPL); or with DLVD but not DLVA (DLVD>>DLVA; activates lacZ andLEU2 only; representative sequence, SGKFGEALPGWLSSACWCFG), and a nonspecific control peptide (negative; activates no reporters; representative sequence, EQLKYNRFWPWQWWGGRRLR). (C) Confirmation that peptides interact with HEF1 in an in vitrosystem, using pulldowns of endogenous HEF1 from cell lysates with the three GST-fused peptides from B, or with GST only. (Top) Levels of GST peptide or GST only in reaction; (bottom) associated HEF1.











