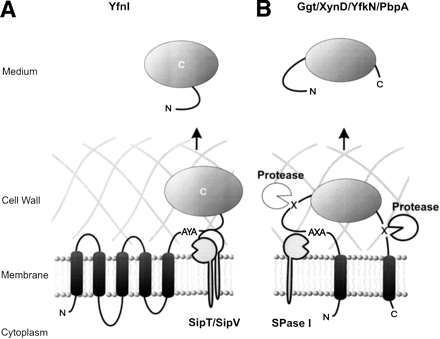
Model for the release of proteins with transmembrane segments. (A) Processing of the polytopic membrane protein YfnI by the type I signal peptidases SipT and SipV at the AYA cleavage site. Cleavage occurs carboxy-terminally of A228 (see also Hirose et al. 2000). (B) Cleavage and release of the YfkN and YhcR proteins, which have a predicted amino-terminal signal peptide and a potential carboxy-terminal transmembrane segment. Cleavage is catalyzed by as yet unidentified signal peptidases and/or proteases that are active at the membrane cell-wall interface. (C) Cleavage and release of PbpA, which has a predicted amino-terminal transmembrane segment and lacks a typical SPase cleavage site.











