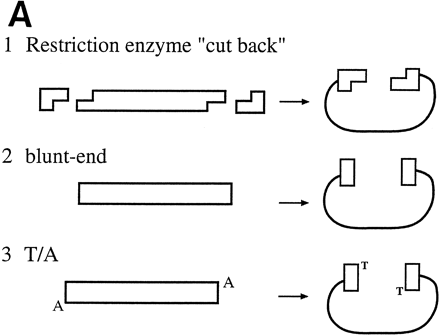
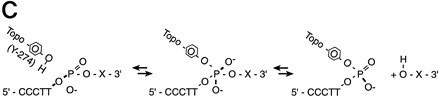
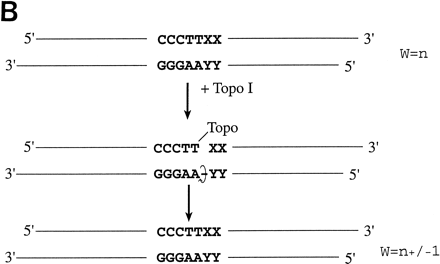
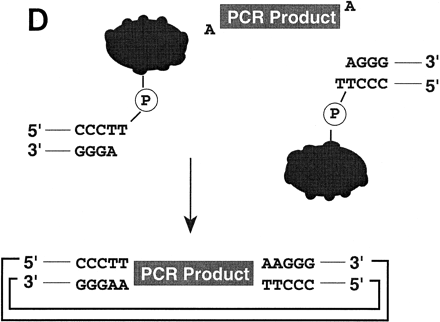
(A) Conventional techniques used to clone PCR products include (1) restriction-cutback methods, in which a restriction endonuclease is used to cleave both DNA insert and vector, leaving complementary overhangs; (2) blunt-end cloning, in which both insert and vector are prepared to have blunt ends; (3) TA Cloning uses the thermophilicTaqI polymerase to add a single 3′A overhang to each end of a PCR product, which can then be joined to a TA Cloning vector with single 3′T overhangs. All three methods involve a second step in which the PCR product and the vector are joined by the action of DNA ligase. (B) During DNA relaxation, the enzyme Vaccinia DNA topoisomerase I cleaves the phosphodiester backbone of one strand at a consensus pentapyrimidine element 5′-(C/T)CCTT in the scissile strand, allowing the DNA to unwind and reduce its winding (W) number,n, to n+1 or n−1 (for DNA that was negatively or positively supercoiled, respectively). (C) In the cleavage reaction, bond energy is conserved by formation of a covalent adduct between the 3′ phosphate of the incised strand and a tyrosyl residue (Tyr-274) of the protein. The covalent complex can reclose across the same bond originally cleaved or it can combine with a heterologous acceptor DNA that has a 5′ hydroxyl tail complementary to that of the adduct, and thereby create a recombinant molecule. (D) Topoisomerase I-mediated cloning uses the reaction mediated by Vaccinia DNA topoisomerase I to join PCR-amplified DNA fragments into plasmid vectors. PCR fragments have 5′ hydroxyl residues from the primers used for the amplification reaction, and therefore are an ideal substrate for the topoisomerase ligation reaction. The topoisomerase I (solid black shape) is shown linked to the vector through the 3′ phosphate (P) of the incised strand. The PCR product has single 3′ A overhangs (A).











