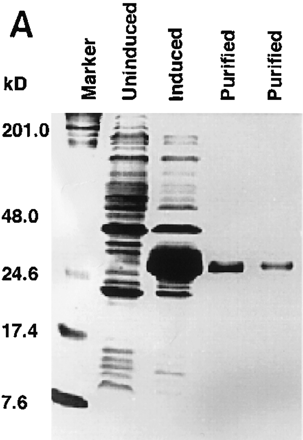
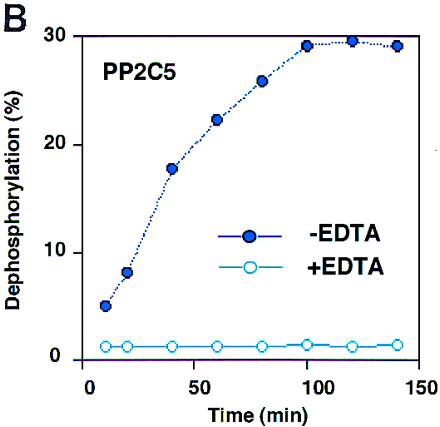
Purification of recombinant protein and enzyme activity assay. (A) Gene expression and protein purification of PP2C5. E. coli containing the recombinant expression plasmid was grown for 3 hr either under inducing or noninducing conditions. Following protein isolation, samples were analyzed on a 12% SDS-polyacrylamide gel. Fromleft to right, the samples were as follows: protein marker (prestained SDS-PAGE standards, Bio-Rad, 25 μg in 20 μl); PP2C5-noninduced total protein (40 μg in 40 μl); PP2C5-induced total protein (40 μg in 40 μl); purified PP2C5 protein (5 μg in 5 μl); and purified PP2C5 protein (2.5 μg in 2.5 μl). (B) Enzymatic activity assay for PP2C. One microgram of purified PP2C5 was used in each reaction. Each experiment was repeated twice. Each value represents the average of the two different experiments. Labeled protein was dephosphorylated at 30°C in a 10-μl solution [containing 50 mm Tris-HCl at pH 7.0, 0.1% (vol/vol) 2-mercaptoethanol, ∼3 × 105 cpm32P-labeled casein, ∼1 μg of recombinant protein, and either 10 mm Mg2+ and 10 mmMn2+ or the same concentration of cations plus 10 mm EDTA]. At each time point (min), 100 μl of 20% trichloroacetic acid (TCA) was added, incubated on ice for 5 min, and microcentrifuged at room temperature for 5 min to precipitate the protein. Radioactivity in 90 μl of supernatant and 20 μl of pellet was measured by scintillation counting. Dephosphorylation was expressed as a percentage of 32P released into the supernatant. (●) −EDTA; (○) +EDTA.











