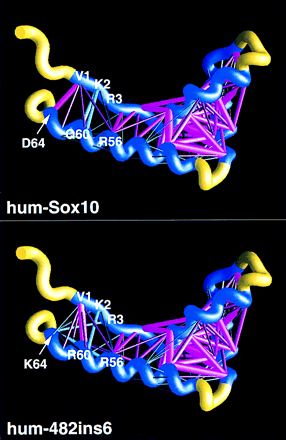
Energy scaffolds for wild-type and mutant Sox10 sequences containing the HMG-1 box domain. The α carbon backbone of the protein is depicted as a curving worm. Within the backbone, segments of the HMG-1 box domain comprising the core folding motif are shown in blue, while the intervening loop regions are shown in yellow. Pairwise residue interaction energies between core residues (Bryant and Lawrence 1993) are indicated by the thickness and coloring of the connecting α carbon positions in the protein backbone. Thick, magenta-colored cylinders are the most favorable interactions; thick, cyan-colored cylinders indicate the least favorable interactions. Intermediate colors and cylinder thicknesses represent interactions falling between these extremes. Numbering corresponds to that in the multiple sequence alignment in Fig. 6 and in Baxevanis et al. (1995). Scaffolds were generated by use of the graphics program GRASP (Nicholls et al. 1991). (Top) Human SOX10; (bottom) human 482ins6 mutant, yielding a Leu–Arg insertion at position 59.











