The Synuclein Family
Abstract
The synuclein gene family recently came into the spotlight, when one of its members, α-synuclein, was found to be mutated in several families with autosomal dominant Parkinson’s disease (PD). A peptide of the α-synuclein protein had been characterized previously as a major component of amyloid plaques in brains of patients with Alzheimer’s disease (AD). The mechanism by which this presynaptic protein is involved in the two most common neurodegenerative disorders, AD and PD, remains unclear. Remarkably, another member of this gene family, γ-synuclein, has been shown to be overexpressed in breast carcinomas and may also be overexpressed in ovarian cancer. The possible involvement of the synuclein proteins in the etiology of common human diseases has raised exciting questions and is the subject of intense investigation. Details of the properties of any member of the synuclein family may provide useful information for understanding the characteristics and function of other family members. The present review offers a synopsis of the current state of knowledge of all synuclein family members in different species.
History
Ten years ago a protein was isolated fromTorpedo californica using an antiserum against cholinergic vesicles; because of its localization to the nuclear envelope of neurons and to presynaptic nerve terminals, this protein was named synuclein (Maroteaux et al. 1988). During investigations of the composition of amyloid plaques of Alzheimer’s disease (AD) patients a peptide was solubilized and thus termed NAC, fornon-Aβ component of AD amyloid; the precursor protein of this peptide named NACP was found to be homologous to the rat α-synuclein (Ueda et al. 1993). Later, a 14-kD phosphoneuroprotein identified in rat and bovine was determined to be a member of the synuclein family (Tobe et al. 1992; Nakajo et al. 1993; Shibayama-Imazu et al. 1993). NACP and the human ortholog of the 14-kD phosphoneuroprotein were identified as two distinct synucleins and termed α-synuclein and β-synuclein, respectively (Jakes et al. 1994). More recently, a new member of the human synuclein family, γ-synuclein, was isolated and characterized (Ji et al. 1997;Lavedan et al. 1998b).
Identification and Classification of Synuclein Sequences
There are currently almost 200 DNA and protein sequences in the sequence databases with high homology to the α-synuclein gene or protein. All synuclein sequences available to date from Homo sapiens, Mus musculus, and Rattus norvegicus can be assigned to three distinct protein groups: α-, β-, and γ-synuclein. Synuclein proteins have also been identified in other organisms: synelfin is the α-synuclein ortholog in Serinus canaria (George et al. 1995), phosphoneuroprotein 14 (PNP14) is the β-synuclein ortholog in Bos taurus (Nakajo et al. 1990), and the first synuclein protein described in T. californicacorresponds to the human γ-synuclein. Interestingly, no homologous proteins have yet been identified in Escherichia coli, Saccharomyces cerevisiae, Caenorhabditis elegans, orDrosophila melanogaster.
The Human Synuclein Genes
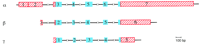
The α-, β-, and γ-synuclein genes (SNCA, SNCB, and SNCG) map to chromosome 4q21.3–q22 (Campion et al. 195; Chen et al. 1995; Shibasaki et al. 1995; Lavedan et al. 1998a), 5q35, (Spillantini et al. 1995; C. Lavedan, E. Leroy, R. Torres, A. Dehejia, S. Buchholtz, A. Dutra, R.L. Nussbaum, and M.H. Polymeropoulos, in prep.) and 10q23 (Lavedan et al. 1998b), respectively. All three genes are composed of five coding exons of similar sizes, and the overall organization of these genes is very well conserved (Fig. 1).
Organization of the human synuclein genes. Exons are represented by boxes, blue or red, for the coding or untranslated regions respectively. Introns (not drawn to scale) are shown as interrupted horizontal lines.
The 5′-untranslated region of the α-synuclein gene contains an exon with two alternative spliced sites, which could be considered as two adjacent exons (exons 1 and 2). Alternative splicing has also been observed for exons 4 and 6 of the α-synuclein gene (Ueda et al. 1994; Campion et al. 1995). Similarly, the three rat cDNAs SYN1, SYN2, and SYN3, appear to be splice variants of the same gene (Maroteaux et al. 1988; Maroteaux and Scheller 1991) with most homology to human α-synuclein. So far no splice variant has been described for the β- and γ-synuclein genes.
The Synuclein Proteins
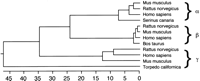
A phylogenic tree of all synuclein proteins known to date (Fig.2) shows that α- and β- synucleins are related more closely to each other than to γ-synuclein. Surprisingly, the torpedo protein, which was originally used to isolate the rat α-synuclein is actually related more closely to γ-synuclein. The synucleins are small proteins of a calculated molecular mass close to 14,000. However, it has been shown that their apparent molecular mass is ∼19–20 KD, indicating that they are post-transcriptionally modified (Jakes et al. 1994).
Phylogenic tree of the synuclein proteins. The distance between sequences is indicated by the number of substitutions. The GenBank accession nos. of the DNA sequences used to predict the protein sequences were L08850 (human α-synuclein), S69965 (human β-synuclein), AF044311 (human γ-synuclein), multiple aligned ESTs (mouse α-, β-, and γ-synuclein), AF007758 (rat α-synuclein), H32092 and D17764 (rat β-synuclein), X86789(rat γ-synuclein), L33860 (canary synelfin). SWISS-PROT accession nos. P33567 (bovine PNP14) and P37379 (torpedo synuclein) were also used.
The α-Synuclein Proteins
The α-synuclein proteins are identical for the first 42 amino acids (Fig. 3). The α-synuclein protein is 140amino acids long in human and rodents, and 143 in S. canaria. The human and rodent sequences are 95.3% identical. One of the only 6-amino-acid differences is the amino acid at position 53, which is normally an alanine in humans and threonine in rodents. Interestingly, it is the same substitution, Ala-53–Thr, that was observed in some familial cases of Parkinson’s disease (PD) (Polymeropoulos et al. 1997). The mouse and rat α-synuclein proteins differ by a single amino acid at position 121. The synelfin protein is 86.7% and 85.3% identical to the human and rodent α-synucleins, respectively. Conformational analysis of the human α-synuclein has led to the speculation that this protein is natively unfolded and therefore could be involved in protein–protein interactions (Weinreb et al. 1996).
Alignment of the synuclein proteins. For each class of protein, residues identical to the consensus sequence are boxed, and the ruler is defined for the human protein. Residues that are conserved in all three classes of synuclein proteins throughout the species are shown in blue. Residues in red are specific to a particular class of synuclein proteins.
The β-Synuclein Proteins
β-Synuclein proteins are known for human, rat, mouse, and bovine. They are 134 amino acids long and are the most conserved of the synuclein proteins (Fig. 3). Notably, they lack 11 amino acids in the region corresponding to the NAC35 peptide found in plaques of AD patients, which extends from residues 61 to 95 of α-synuclein. Mouse and rat β-synuclein proteins are identical and share 97.8% identity with the human β-synuclein protein. The bovine PNP14 is 97% and 98.5% identical to the human and rodent β-synuclein, respectively. Nakajo and colleagues have shown that β-synuclein is phosphorylated in the rat brain, presumably by the C2+calmodulin protein kinase II (Nakajo et al. 1993). A possible phosphorylation site is the serine residue at position 118 (Nakajo et al. 1993), which is conserved throughout species in the β-synuclein sequence but not in the α- or γ-synuclein.
The γ-Synuclein Proteins
The γ-synuclein protein is the least conserved of the synuclein proteins (Fig. 3). The human γ-synuclein is 127 amino acids long, and is 87.7% and 83.8% identical to the mouse and rat proteins, respectively, which are 4 amino acids shorter. As for the α- and β-synuclein proteins, the region of highest homology is the amino-terminal region.
The torpedo synuclein protein is 143 amino acids long, including a duplication (KTKQGVQDAAE) not present in the other synuclein proteins. Besides this duplication, the torpedo synuclein is 75.3% identical to the amino-terminal portion of the human γ-synuclein. The last 35 amino acids have little homology with the human or rodent γ-synucleins.
Repeated Domains
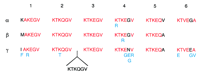
The synuclein proteins contain several repeated domains that display variations of a KTKEGV consensus sequence (Fig.4). The β-synuclein protein contains five of these domains, whereas the α- and γ-synucleins have six, with the exception of the torpedo γ-synuclein, which has seven. The very high conservation between species for a specific repeated domain of a particular protein suggests that the repeated domains have arisen from the duplication of a single domain within an ancestral synuclein gene. Later, this ancestral gene may have undergone successive duplications to give rise to the three synuclein genes in which the repeated domains may still be able to diverge. The third domain, however, remained absolutely identical, KTKEGV, in all genes throughout all species (Fig.4). It was originally noticed by Maroteaux et al. (1988), that the same type of domain is present in proteins of the rho family. However, as of today, the role of these domains remains unknown.
Comparison of the repeated domains in the synuclein proteins. Repeated domains are numbered from 1 to 6, from the amino to the carboxyl terminus. For each class of synuclein, the residues of the human protein are shown with the changes observed in other species indicated below in blue. For each domain, the consensus residues for the three human synucleins are shown in red. Note the insertion of an additional domain in T. californica, between domains 2 and 3.
Another type of organization of the synuclein proteins, with an 11-residue repeated motif, has been proposed (George et al. 1995). This motif, repeated six to seven times in the amino-terminal portion of the protein, is reminiscent of the amphipathic α-helical domains of the apolipoproteins (Segrest et al. 1990; Segrest et al. 1992) and suggests lipid binding properties (George et al. 1995; Davidson et al. 1998).
Tissue Expression of the Synuclein Proteins
The expression of the synuclein genes has been studied mainly by Northern and Western blot analysis. Because of the high homology between the three synuclein family members, caution must be used in interpreting results of experiments in which the specificity of the probe used (cDNA or antibody) has not been demonstrated; this is particularly true for earlier studies, in which the different members of the synuclein family had not yet been identified.
Tissue Distribution
All three human synuclein genes are highly expressed in brain; in the adult rat brain, β-synuclein was reported to be very abundant, as much as 0.1% of the protein in the homogenate (Shibayama-Imazu et al. 1993). Other tissues show lower levels of expression (Table1). The distribution of the proteins within the brain is however somewhat different (Table 2): α- and γ-synuclein seem more widely expressed than β-synuclein. The three human synuclein genes are expressed in the thalamus, the substantia nigra, the caudate nucleus, and the amygdala. Only γ-synuclein appears strongly expressed in the subthalamic nucleus.
Tissue Expression of the Human Synuclein Genes
Expression of the Human Synuclein Genes in the Brain
Studies in a hematopoietic cell line indicate that α-synuclein expression is up-regulated during phorbol ester-induced megakaryocytic differentiation, while β-synuclein is down-regulated (Hashimoto et al. 1997). Also α-synuclein but not β-synuclein is found to be abundant in platelets (Hashimoto et al. 1997).
Localization of the Synuclein Proteins
Studies of the electric organ of the torpedo originally indicated that synuclein was localized in presynaptic nerve terminals (Maroteaux et al. 1988). This localization was later confirmed in rat (Nakajo et al. 1990; Tobe et al. 1992; Shibayama-Imazu et al. 1993; Jakes et al. 1994; Iwai et al. 1995a), zebra finch (George et al. 1995; Withers et al. 1997), and human (Jakes et al. 1994). Both α-synuclein and β-synuclein are found predominantly in nerve terminals, in close proximity to synaptic vesicles (Shibayama-Imazu et al. 1993; Jakes et al. 1994; Iwai et al. 1995a). Immunohistochemical studies have revealed that in the adult human entorhinal cortex, α- and β-synuclein are both expressed strongly in the gray matter (Jakes et al. 1994): α-Synuclein staining appears granular and concentrated around nerve cell bodies, whereas β-synuclein staining seems more fibrous (Jakes et al. 1994). However, the cellular localization of α-synuclein seems to change during the development of the central nervous system;Hsu et al. (1998) have observed in rat that α-synuclein is present in the cell body of neuronal precursors and is later redistributed to the nerve terminals. The torpedo synuclein was initially reported to be localized in portions of the nuclear envelope (Maroteaux et al. 1988), but studies in rat and human brains showed that both α- and β-synuclein were mainly in the cytosol (Tobe et al. 1992; Irizarry et al. 1996). It is not clear whether this difference of localization could be explained by the fact that the torpedo synuclein shares less homology with α- and β-synuclein than with γ-synuclein for which the localization has not yet been determined.
Sequence Databases
In addition to the identification and classification of homologous genes and proteins, the analysis of sequence databases may reveal information on the expression of these genes. Although caution must be observed in interpreting such data, the identification of the tissues from which the cDNA libraries were made can reveal when and where a particular gene may be expressed. Moreover, unexpected results may indicate new avenues of research. In this regard, the analysis of the synuclein sequences present in the databases is interesting.
For example, among the 64 human α-synuclein expressed sequence tags (ESTs) reported, 19 originated from brain libraries: 100% (19/19) from fetal or infant brain libraries, but none from adult brain libraries. For β- and γ-synuclein the proportion of ESTs from adult brain is 67% (8/12) and 100% (4/4), respectively. Furthermore, >68% of human α-synuclein ESTs originated from fetal or infant tissues, whereas the proportion is only ∼20% for β- and γ-synuclein. This observation indicates that in addition to being expressed in the adult (Ueda et al. 1993), α-synuclein may be highly expressed early in development. In rodent brain, the expression of α- and β-synuclein is increased dramatically at later stages of embryonic and early postnatal development (Hashimoto et al. 1997; Hong et al. 1998; Hsu et al. 1998).
Another striking observation is that 53% (10/19) of the human γ-synuclein sequences originated from tumor tissues, including 37% (7/19) from an ovarian tumor library (NbHOT). This suggests that γ-synuclein, which is overexpressed in some breast carcinomas (Ji et al. 1997), may be overexpressed in other tumors, particularly ovarian tumors (Lavedan et al. 1998b).
Synuclein Protein Function
A very important clue as to the role of the synuclein proteins came recently from the identification and the characterization of a specific phospholipase D isoform that localizes to the plasma membrane: phospholipase D2 (PLD2) (Colley et al. 1997). Because PLD2 appears to induce cortical reorganization that results in directed movement of vesicles, it was suggested that it may have a role in signal-induced cytoskeletal regulation and/or endocytosis (Colley et al. 1997). Subsequently, Jenco et al. reported that α- and β-synuclein may be able to selectively inhibit PLD2, possibly by direct interaction at the membrane surface (Jenco et al. 1998). Therefore, it is possible that synucleins are important regulatory components of the vesicular transport processes (Jenco et al. 1998). Remarkably, Maroteaux and Scheller (1991) had originally noticed that α-synuclein expression in the rat brain (cerebral cortex, hippocampus, dentate gyrus) correlates with the localization of molecules involved in the phosphoinositol (PI) second messenger pathway such as phospholipase C1 and the muscarinic cholinergic receptors type m1 and m3. This finding led them to hypothesize that α-synuclein may be implicated in synaptic vesicle release and/or recycling in response to PI stimulation. This attractive idea is supported by the fact that α-synuclein has been shown to bind small unilamellar phospholipid vesicles (Davidson et al. 1998) and also that various components of the cytoskeleton including neurofilament proteins have been detected in neuronal inclusions (Iwatsubo et al. 1996) that contain α-synuclein (see below). This hypothesis is also in agreement with earlier studies implicating synelfin in the canary song-learning process via the regulation of neural plasticity (George et al. 1995).
The observation of increased expression of β-synuclein in rat Sertoli cells, from 9 to 26 days after birth, has suggested that it may have a role in the meiosis of spermatogonia (Shibayama-Imazu et al. 1998). In these cells, β-synuclein appears to be associated with fibrillar structures, which have not yet been characterized (Shibayama-Imazu et al. 1998).
Because of the high species conservation among family members, animal models such as knockout and transgenic mice may provide additional information to the normal function and possible interrelationship of the synuclein proteins.
Synuclein Proteins and Human Diseases
γ-Synuclein in Tumorigenesis
The possible involvement of γ-synuclein in tumorigenesis first came to light when a gene named BCSG1 (breastcancer-specific gene1) was shown to be overexpressed in advanced infiltrating carcinoma of the breast (Ji et al. 1997). In fact, BCSG1 and γ-synuclein appear to be the same protein (Lavedan et al. 1998b). What role this protein has in normal breast tissue and how it is implicated in breast and perhaps in ovarian cancer remains a mystery. In particular, the possible inhibitory effect of γ-synuclein on PLD2 remains to be investigated.
α-Synuclein in the Brains of AD and PD Patients
Both AD and PD are characterized by abnormal protein deposition in various areas of the brain. In AD, the β-amyloid protein (Aβ) is converted into amyloid fibrils, which are the major component of the neuritic plaques. The discovery that a portion of α-synuclein was a constituent of the amyloid plaques of AD patients (Ueda et al. 1993) has prompted further research into its possible involvement in the pathogenesis of AD. In particular, accumulation of α-synuclein in presynaptic terminals and dystrophic neurons of AD patients (Masliah et al. 1996), as well as increased levels of α-synuclein in the frontal cortex at the early stages of AD (Iwai et al. 1996), have been observed. Interestingly, several studies have demonstrated that the NAC peptide of α-synuclein was not only prone to self-aggregation in vitro and could form amyloid fibrils (Iwai et al. 1995b; Jensen et al. 1997; Hashimoto et al. 1998; Paik et al. 1998) but was also able to seed Aβ polymerization (Han et al. 1995; Yoshimoto et al. 1995;Paik et al. 1998). Similarly, NAC amyloid formation can be seeded by either the β peptide or the prion protein PrP (Han et al. 1995). Furthermore, it has been suggested that changes in the α-synuclein protein might promote α-synuclein self-aggregation and lead to the formation of amyloid-like structures (Polymeropoulos et al. 1997).
It has been shown that α-synuclein accumulates frequently in fibrous cytoplasmic inclusions known as Lewy bodies and in abnormally shaped neurites known as Lewy neurites, found in brains of PD patients even though they do not carry α-synuclein mutations (Spillantini et al. 1997, 1998; Wakabayashi et al. 1997; Baba et al. 1998; Hashimoto et al. 1998; Irizarry et al. 1989; Mezey et al. 1998b; Takeda et al. 1998). Spillantini et al. (1998) have shown more precisely that α-synuclein molecules form the major component of Lewy bodies and Lewy neurites. α-Synuclein appears to be a more sensitive marker of these inclusions than ubiquitin, which was used previously (Mezey et al. 1998b; Spillantini et al. 1998). However, even though our knowledge of the composition of the Lewy bodies has advanced, the involvement of these inclusions in the specific neuronal death observed in PD and their role in the pathogenesis of the disease are still enigmatic. The mechanism by which α-synuclein accumulates in Lewy bodies is not understood. It is possible that normal α-synuclein aggregates because of overexpression of the protein, and/or by binding to other proteins. Alternatively, damage to α-synuclein, for example, by oxidation, free radicals, or elevated temperature, may lead to protein aggregation; it has been shown that high temperature stimulates the aggregation of normal α-synuclein in vitro (Hashimoto et al. 1998). Could exposure to high temperatures, such as fevers, be a risk factor for developing PD?
α-Synuclein Accumulation in Other Neurodegenerative Disorders
Interestingly, the cerebral cortex of patients with a common late onset type of dementia similar to AD, known as diffuse Lewy Body disease (DLBD), is characterized by the presence of Lewy bodies and Lewy neurites, that contain α-synuclein (Mezey et al. 1998b;Spillantini et al. 1998). α-Synuclein is also a component of the glial cytoplasmic inclusions found in the brain of patients affected with multiple system atrophy (MSA) and amyotrophic lateral sclerosis (ALS) (Mezey et al. 1998a). Importantly, the fact that inclusion bodies observed in other disorders, such as Pick’s disease and multi-infarct dementia, do not seem to contain α-synuclein indicates that aggregation of α-synuclein is not just the consequence of nonspecific neuronal damage (Mezey et al. 1998b).
β- and γ-Synucleins have not been detected in Lewy bodies or Lewy neurites (Spillantini et al. 1998). Further studies will have to be conducted to determine whether or not they participate to the formation of other neuronal inclusions.
The list of diseases with α-synuclein-positive inclusions is certainly not complete, and it would be interesting to investigate whether other disorders with ubiquitin-positive inclusions, such as Huntington’s disease and Machado–Joseph disease, are also part of the “synucleinopathies” (Mezey et al. 1998a).
Molecular Analysis
Molecular analysis of α-synuclein has been performed on a number of AD families, but no association or linkage betweenSNCA and the disease could be demonstrated (Xia et al. 1996), suggesting that this gene may not have a direct role in the genetics of AD. However, population studies have led to the speculation that a particular dinucleotide repeat allele close to SNCA may have a protective function for AD (Xia et al. 1996).
Two different mutations in SNCA have been reported associated with PD in a few families in which the disease is transmitted in an autosomal dominant manner. These two mutations, Ala-53–Thr (Polymeropoulos et al. 1997) and Ala-30–Pro (Kruger et al. 1998) appear to account only for a minority of PD cases (Munoz et al. 1997;Polymeropoulos et al. 1997; Bennett and Nicholl 1998; Chan et al. 1998;Farrer et al. 1998; Kruger et al. 1998; Vaughan et al. 1998; Zareparsi et al. 1998). However, as discussed above, the abundance of α-synuclein in Lewy bodies and Lewy neurites of sporadic PD patients with no α-synuclein mutation suggests that this protein has an important role in the pathogenesis of the disease. Therefore, the identification and analysis of even rare α-synuclein mutations, perhaps in regulatory elements that have not yet been identified, or at the transcript level as seen in the β-amyloid precursor in AD patients (van Leeuwen et al. 1998), may provide clues as to how the protein is involved in the development of PD.
Conclusion
The discovery of mutations in α-synuclein associated with PD has revitalized the interest in a genetic contribution to this disorder (Nussbaum and Polymeropoulos 1997), and in the hypothesis of abnormal protein aggregation as a cause of neuronal death (Lansbury 1997). The amyloidogenic properties of α-synuclein, which may promote protein aggregation and deposition, are reminiscent of what has been observed for other proteins in several other neurodegenerative disorders, including AD, Huntington’s disease, and prion diseases (Lansbury 1997). The observation that α-synuclein accumulates in Lewy bodies should refocus research efforts in determining the composition of neuronal inclusions, which have been at times regarded only as “waste baskets.” A better characterization of their components and a careful study of their formation may be critical in identifying new candidate genes for neurodegenerative disorders and new drug targets.
The surprising involvement of γ-synuclein in carcinogenesis will draw additional attention to this protein family. More studies are certainly needed to determine how γ-synuclein is overexpressed in tumors (DNA amplification or enhanced transcription) and whether it is the primary cause of tumor development or a response to a preceding event.
The fact that synuclein proteins may be able to inhibit PLD2 (Jenco et al. 1998) and could bind small vesicles (Davidson et al. 1998) opens a vast field of investigation that may implicate the complex PI pathway and cytoskeletal proteins. Substantial work is required to characterize the type of association between the synuclein proteins and vesicles. In particular, the identification of the lipid and/or protein partners of the synuclein proteins may be essential to clarify their contribution, if any, to the control of vesicular transport processes (Jenco et al. 1998).
The molecular dissection of how synucleins are involved in human diseases is an exciting and challenging endeavor that should lead to a better understanding of neuronal degeneration, and perhaps carcinogenesis. These studies should provide new tools to aid in elucidating the pathogenesis of these diseases and should ultimately lead to the development of more effective therapies.
Acknowledgments
I thank Drs. Robert L. Nussbaum and Mihael H. Polymeropoulos for their advice, discussion, and critical review of the manuscript. I am also grateful to Dr. Elisabeth Leroy for her comments and for sharing unpublished information on the tissue expression of the human synuclein genes and to Dr. Sharon F. Suchy for her support and her helpful reading of the manuscript.
NOTE ADDED IN PROOF
The localization of SNCG to chromosome 10q23 was confirmed by Ninkina et al. (1998), who named that genepersyn. The persyn protein appears to influence neurofilament network integrity by increasing the susceptibility of NF-H to calcium-dependent proteases (Buchman et al. 1998).
Buchman, V.L., J. Adu, L.G.P. Pinõn, N.N. Ninkina, and A.M. Davies. 1998. Persyn, a member of the synuclein family, influences neurofilament network integrity. Nat. Neurosci. 1: 101–103.
Ninkina, N.N., M.V. Alimova-Kost, J.W.E. Paterson, L. Delaney, B.B. Cohen, S. Imreh, N.V. Gnuchev, A.M. Davies, and V.L. Buchman. 1998. Organization, expression and polymorphism of the human persyn gene. Hum. Mol. Genet. 7: 1417–1424.
Footnotes
-
↵1 E-MAIL ; FAX (301) 402-2170.
- Cold Spring Harbor Laboratory Press