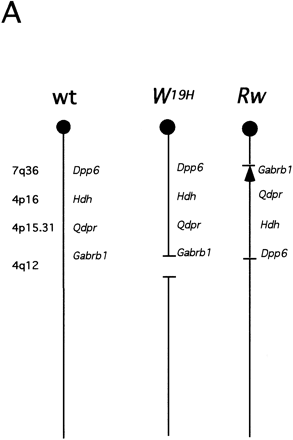
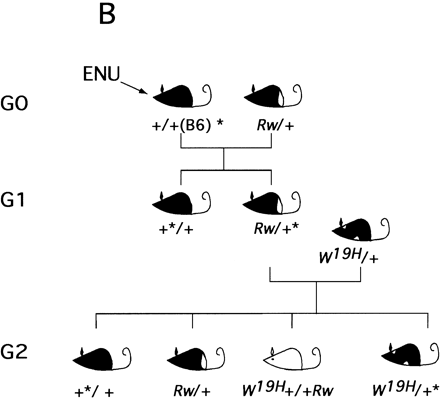
Region-specific mutagenesis screen for novel mutations in the proximal portion of mouse chromosome 5. (A) Schematic representation of the wild-type chromosome 5 and the two marker chromosomes Rwand W19H. The approximate position of four loci that serve as starting points for the generation of deletion complexes (dipeptidyl amino peptidase like protein 6, Dpp6; mouse homolog of the Huntington disease gene, Hdh; quininoid dihydropteridine reductase, Qdpr; and GABAAreceptor, β-1 subunit, Gabrb1) are indicated. Atleft chromosomal locations of these four loci in the human genome are indicated. The Rw chromosome is associated with a 30-cM chromosomal inversion (Stephenson et al. 1994; R.B. Hough, A. Lengeling, and M. Bucan, unpubl.). The W19H mutation is associated with a 4-Mb chromosomal deletion, which includes several known genes, such as a cluster of receptor tyrosine kinases, Kit, Pdgfra, and Flk1, cytoplasmic kinases Txk andTec, and the Clock gene (King et al. 1997a,b). (B) The breeding scheme of the pilot screen usingW19H. In this screen, ENU-treated males are crossed to Rw/+ females and the Rw/+ G1progeny are used for matings with the W19H /+ mice to generate G2 families that are screened for novel (recessive) mutations within the chromosomal region uncovered by theW19H deletion. Four classes of (G2) progeny are generated in this cross. (1) Mice with theW19H deletion in trans to the mutagenized chromosome; (2) mice compound heterozygous for W19H deletion and Rw chromosome; (3) normally pigmented mice that are heterozygous for mutagenized chromosome; and (4) mice heterozygous for the Rw chromosome. W19H /+* mice are observed for novel recessive mutations; failure to recoverW9H /+* mice in this cross implies that a recessive lethal mutation exists in this region.











