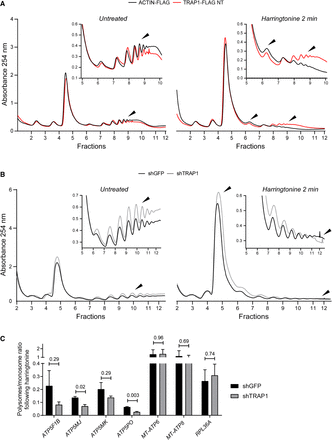
TRAP1 slows down translation elongation by cytoplasmic ribosomes. (A,B) Polysome profiling absorbance, measured at 254 nm, of extracts from ACTIN-FLAG (control) and TRAP1-FLAG overexpressing HeLa cells (A) and from shGFP (control) and shTRAP1 HeLa cells (B), 24 h (A) or 72 h (B) after induction, in the absence (left) or presence (right) of 2 µg/mL of harringtonine (cells were treated for 2 min and then blocked with cycloheximide). Inset areas show magnification of regions of interest. (C) Ratio between polysome-associated and monosome-associated mRNAs following harringtonine treatment (2 µg/mL, 2 min), normalized on the respective untreated samples. The amount of the associated transcripts was measured by RT-qPCR performed on RNAs extracted from pooled monosomal and polysomal fractions, both corrected for an external reference spike-in RNA (luciferase). Data are represented as mean ± SEM from three independent experiments. Numbers above bars represent the statistical significance (P-value) calculated by a multiple t-test.











