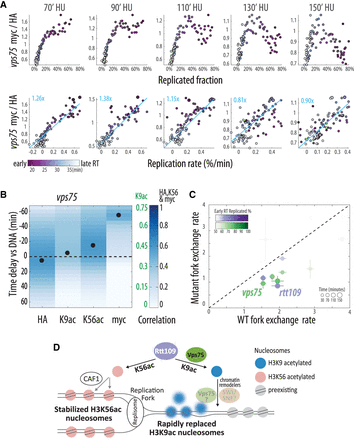
N-terminal H3 acetylation mediates histone exchange ahead of the fork. (A–C) VPS75-deleted cells lose replication-associated K9ac and display reduced H3 exchange ahead of the fork but maintain stable nucleosomes behind it. (A,B) As in Figure 2C and 2A, respectively, for VPS75-deleted cells (n = 2) (see also Supplemental Figure S4). HA data for times 50 and 90 were interpolated (see Methods). Of note is the delayed H3K9 acetylation (B; cf. to wild-type cells Fig. 2A; Supplemental Fig. S1A). Note the different color scales for the epitopes. (C) As in Figure 3C with the addition of the vps75 mutant. (D) Rtt109's dual role in regulating histone exchange during DNA replication. A schematic model describing the main findings: Rtt109-dependent acetylation of K56 stabilizes nucleosomes behind the replication fork, whereas acetylation of K9 promotes nucleosome replacement ahead of the fork. See Discussion.











