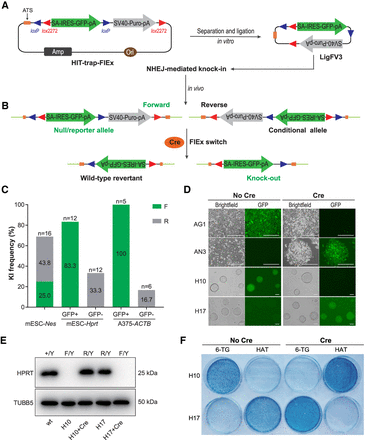
Combining HIT-trapping and FlEx switch enabled the simultaneous generation of reversible and conditional knockouts. (A,B) Schematic showing the workflow and mechanism for simultaneous generation of reversible and conditional knockouts at target sites. Optimized donor LigFV3 was generated through self-ligation of the insertion cassette, excised from the plasmid HIT-trap-FlEx in vitro. Because of the nondirectional feature of NHEJ-mediated knock-in, cells containing either forward or reverse integration can be obtained in a single transfection step. The Cre-mediated FlEx switch enabled the inversion of the gene-trap cassette post insertion, leading to phenotype reverted or conditional knockout. Blue triangles indicate loxP sites; red triangles, lox2272 sites. (C) Knock-in efficiencies of optimized donor LigFV3 at various target sites. (F/R) Forward/reverse integration of the donor; (n) number of screened clones. The genotyping and Sanger sequencing data are presented in Supplemental Figure S11. (D) Images of A375 cells and mESCs bearing FlEx-trap insertions transfected with a Cre expressing plasmid. (AG1/AN3) A375 cell clones with donor insertion at ACTB locus in forward/reverse orientation; (H10/H17) mESC clones with donor insertion at Hprt locus in forward/reverse orientation. Scale bar, 50 µm. (E) Western blot confirmation of the wild-type revertant and conditional knockout phenotypes of Hprt after exposure to Cre in mESCs, with TUBB5 as the loading control. (Y) Y Chromosome (Hprt locus is located on the X Chromosome). (F) Methylene blue staining of mESCs mentioned above. mESCs were cultured with 6-TG (2 μM) or 1× HAT supplement for 5 d and were fixed and stained with 0.02% methylene blue.











