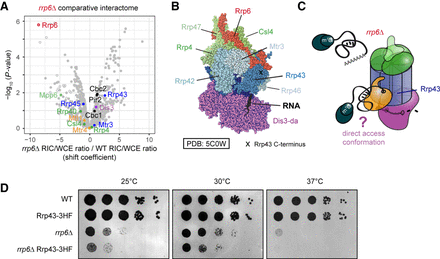
Altered substrate access to the exosome complex in rrp6Δ. (A) Some exosome subunits show increased association with poly(A)+ RNA in rrp6Δ. Volcano plot of a comparative RIC experiment for rrp6Δ as in Figure 4C. (B) Structure of the S. cerevisiae nuclear exosome complex in the direct access conformation (PDB: 5C0W) (Makino et al. 2015). Positions of various subunits and of the C-terminus of Rrp43 are marked. (C) In the absence of Rrp6, nuclear RNPs accumulate but fail to engage with the exosome cap region. At the same time, increased RNA crosslinking with Dis3 and Rrp43 could suggest a potential rerouting of poly(A)+ RNA substrates from the channeling route to direct access. (D) In a plate-based growth assay, addition of a C-terminal tag to Rrp43 results in a slow growth phenotype when combined with rrp6Δ, but not in a wild-type background. Serial dilutions (1:10) of the indicated yeast strains were spotted on YES and incubated at the indicated temperatures for several days.











