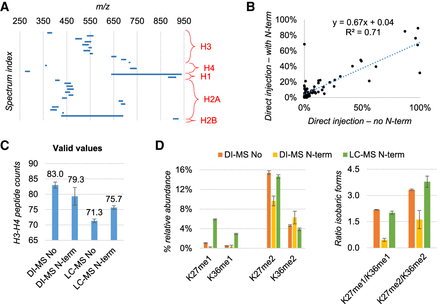
Evaluation of detection biases by skipping peptide N-terminal derivatization. (A) Overview of the full MS acquisition windows set up for the DI-MS method. The MS/MS scans of the isobaric forms are not displayed. (B) Relative abundance of endogenous peptides extracted from human HEK293T cells quantified by DI-MS after excluding (x-axis) or including (y-axis) N-terminal peptide derivatization in the protocol. Data are the average of three biological replicates. (C) Number of peptides quantified from histone H3 and H4 with or without N-termini derivatization by DI-Ms and LC-MS. As expected, without N-termini derivatization LC-MS has more issues in binding and resolving histone peptides. (D) Example of isobaric peptides with relative ratio estimated by MS/MS in DI-MS acquisition. The example was selected specifically because these peptides are baseline resolved by LC-MS, which was used as a reference. The ratio was wrongly estimated by DI-MS when using N-terminal derivatization (right).











