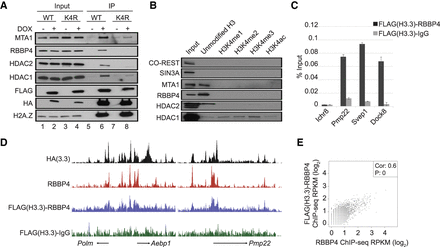
H3.3 interacts with the NuRD complex, and together, H3.3 and NuRD co-occupy many sites in the genome. (A) Validation of H3.3-NuRD interactions by co-immunoprecipitation. Lysate was obtained from MEF cells treated with (+) and without (−) doxycycline. HA/FLAG-H3.3 and HA/FLAG-H3.3K4R were immunoprecipitated with M2-FLAG resin prior to immunoblotting with antibodies specific to NuRD subunits. Five percent of total lysate was used as input. (B) Avidin-H3 N-tail peptide, either unmodified or modified at lysine 4, was incubated with MEF cell extract followed by SDS-PAGE with antibodies specific to various NuRD subunits. Five percent of total lysate was used as input. (C) Sequential FLAG-H3.3-RBBP4 and FLAG-H3.3-IgG ChIP-qPCR assays. Each qPCR reaction was performed in triplicate, and enrichment was normalized to input. Values are presented as means ± SE. (D) Mapped reads from individual HA-H3.3 and RBBP4 ChIP-seq assays (top two lanes) as well as sequential FLAG-H3.3-RBBP4 and FLAG-H3.3-IgG ChIP-seq assays (bottom two lanes). (E) Correlation plots illustrating the relationship between FLAG-H3.3-RBBP4 and RBBP4 ChIP-seq levels across the genome.











