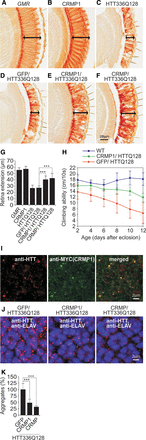
CRMP1 overproduction mitigates mutant HTT-induced photoreceptor degeneration and motor impairment in HD transgenic flies. (A–F) Investigation of eye sections. Analysis of eye sections in control flies (A) and transgenic flies overproducing CRMP1 (B) or HTT336Q128 (C) alone. Coproduction of the control protein GFP does not influence HTT336Q128-induced retina degeneration (D), whereas coproduction of human CRMP1 (E) or Drosophila CRMP (F) mitigates HTT336Q128-induced retinal degeneration. Arrows indicate retinal degeneration. (G) Quantification of retinal degeneration in control and HD transgenic flies (A–F): n ≥ 9 flies per genotype; (***) P ≤ 0.001, two-sided t-test with unequal variance. (H) CRMP1 significantly improves climbing abilities of HTT336Q128 overproducing HD transgenic flies. Data were analyzed using Graphpad Prism 5: n ≥ 16 flies per genotype; P = 0.0017; linear regression followed by F-test. (I–J) Immunofluorescence analysis of eye imaginal discs of HD transgenic flies. (I) MYC-CRMP1 colocalizes with HTT336Q128 in multiple inclusion bodies with a diameter of 0.2–0.4 μm. (J) In contrast to the control protein GFP, coproduction of the proteins CRMP1 and CRMP reduced both the number as well as the average size of HTT336Q128 inclusion bodies. (K) Quantification of the number of HTT336Q128 inclusion bodies in HD flies coproducing modulator proteins. An area of 35.71 μm2 in the middle of the posterior third of eye imaginal discs was systematically analyzed: n ≥ 5; (***) P ≤ 0.001, two-sided t-test with unequal variance. Data in G, H, and K are represented as mean ± SEM.











