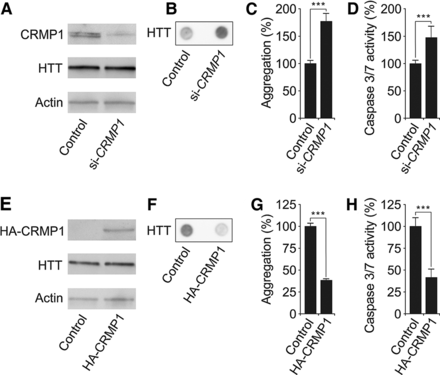
CRMP1 protein production influences polyQ-mediated HTT aggregation and toxicity in PC12 cells. (A,E) SDS-PAGE and immunoblot analysis of PC12 cells overproducing HTTQ103-EGFP fusion protein. Cells treated with si-CRMP1 RNA (A) or overproducing a HA-tagged CRMP1 protein (E) were analyzed. (B,F) Detection of insoluble HTTQ103-EGFP aggregates in si-CRMP1-treated (B) and HA-CRMP1 overproducing (F) PC12 cells. Insoluble aggregates (black dots) were detected by filter retardation assay. (C,G) Quantification of insoluble HTTQ103-EGFP aggregates shown in B and F by image analysis: n = 3; (***) P ≤ 0.001, two-sided t-test. (D,H) Quantification of caspase-3/7 activity in si-CRMP1-treated (D) and HA-CRMP1 overproducing (H) PC12-HTTQ103-EGFP cells: n = 3; (***) P ≤ 0.001, two-sided t-test. Data in C, D, G, and H are represented as mean ± SEM.











