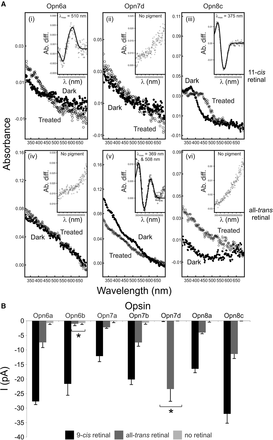
Functional analyses of representative zebrafish new opsins by UV-visible spectroscopy (to determine spectral sensitivity) and whole-cell electrophysiology (to assay phototransduction cascade activation). (A) Diverse absorption spectra of regenerated zebrafish Opn6a (i,iv), Opn7d (ii,v), and Opn8c (iii,vi) photopigments with 11-cis (i–iii) and all-trans (iv–vi) retinal chromophores. For all photopigments, dark (closed circles; i–iii [11-cis retinal] and iv–vi [all-trans retinal]) and light-bleached or acid-treated (open circles) spectra are shown. (Inset) Difference spectra were fitted with a Govardovskii A1-template to determine the λmax value, with x- and y-axes showing the wavelength (λ [nm]) and absorbance difference (Ab. diff.), respectively. (B) Quantification of novel opsin-dependent, light-evoked currents (mean ± SEM) by whole-cell patch-clamp electrophysiology. Recordings were measured from Neuro-2a cells expressing representative zebrafish new photopigments (Opn6a, Opn6b, Opn7a, Opn7b, Opn7d, Opn8a, and Opn8c) preincubated with 9-cis retinal (n = 5–7 cells; black) or all-trans retinal (n = 5–7 cells; dark gray) and exposed to a 10-sec light pulse (420 nm; 8 × 1014 photons/cm2/s1). Data show that these new pigments encode functional photopigments in vitro that interact differently with multiple chromophores, with some pigments being monostable (Opn6b and Opn7d) and others being bistable (Opn6a, Opn7a, Opn7b, Opn8a, and Opn8c). Compared with controls (cells with no retinal; n = 5–7 cells; light gray), all light-evoked currents were significantly greater (P < 0.05, Student's t-test), except for Opn6b preincubated with all-trans retinal and Opn7d preincubated with 9-cis retinal (shown by an asterisk).











