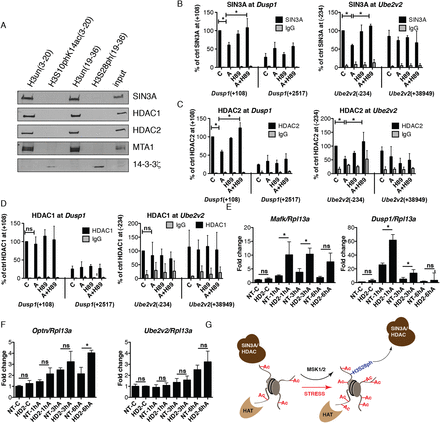
H3S28ph mediates the dissociation of HDAC-containing complexes from target promoters. (A) Western blot analysis of histone pull-down assays with nuclear extracts from HeLa cells treated with anisomycin for 1 h and synthetic peptides corresponding to aa 3–20 and 19–36 of histone H3, either unmodified or carrying the phosphorylation mark at S10 or S28. The association of SIN3A, HDAC1, HDAC2, MTA1, and 14-3-3 zeta (encoded by Ywhaz) with differentially modified peptides was analyzed. (B–D) ChIP-qPCR analysis of changes in SIN3A, HDAC2, and HDAC1 occupancy at Dusp1 and Ube2v2 genes in control (C) and anisomycin-treated cells (A) in the absence and presence of H89. Error bars represent SDs (n = 3). (*) P < 0.05. (E,F) RT-qPCR analysis of Dusp1, Mafk, Ube2v2, and Optn gene expression upon anisomycin treatment (A) in control (NT) and Hdac2 (HD2) knockdown cells. Error bars represent SDs (n = 3). (*) P < 0.05. (G) A model demonstrating the impact of H3S28 phosphorylation on the local histone acetylation levels at stress-induced genes. Local histone acetylation results from the dynamic interplay between recruited HAT and HDAC activities. Upon stress stimulation, MSK1/2 phosphorylates S28 at histone H3 at stress target promoters. This leads to dissociation of HDAC-containing complexes, thereby inducing an increase in local histone acetylation levels.











