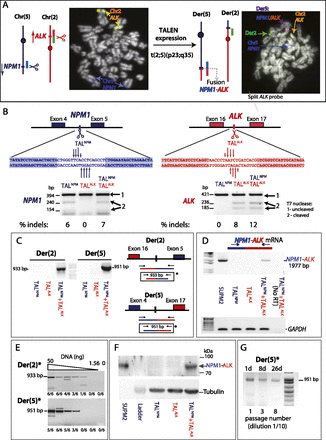
Induction of t(2;5)(p23;q35) translocations with TALENs. (A) ALCL translocations have breakpoints within the NPM1 and ALK genes on chromosomes 2 and 5, respectively, creating an NPM1–ALK fusion gene on der(5). To induce t(2;5)(p23;q35), TALENs are expressed to create DSBs (scissors) in both genes. FISH demonstrates the t(2;5)(p23;q35) translocation after TALEN expression in RPE-1 cells. Red and green signals are from an ALK probe that “breaks apart” upon translocation. The blue signal is from an NPM1 probe. Of 70 metaphases screened, two exhibited translocations and three showed breaks with the ALK break-apart probe, likely due to remaining TALEN expression at this time. (B) TALNPM and TALALK cleavage within NPM1 and ALK introns, respectively, relevant to the NPM1–ALK translocation. DNA binding domains of TALENs are designed to bind the shaded sequences. (Arrows) DSB sites with different tail lengths representing the efficiency of cleavage in vitro, as assayed by in vitro expression of the TALENs (see Supplemental Fig. S6). TALEN cleavage activity in Jurkat cells is monitored by the T7-endonuclease assay, as shown below the sequences. (C) Nested PCR to detect derivative chromosomes der(2) and der(5) in Jurkat cells. Translocation breakpoint junctions are only detected after expression of both TALNPM and TALALK. (D) RT-PCR detection of the NPM1–ALK fusion transcript after TALNPM and TALALK expression in Jurkat cells and in ALCL cell line SUP-M2. The forward primer is within exon 2 of NPM1, and the reverse primer is within exon 29 of ALK, amplifying most of the NPM1-ALK coding sequence. (E) Single-round PCR to detect derivative chromosomes der(2) and der(5) in Jurkat cells. Translocation breakpoint junctions are detected after expression of both TALNPM and TALALK by PCR of the fragment marked (*) in C on serial dilutions of genomic DNA (50, 25, 12.5, 6.25, 3.125, and 1.56 ng). The number of times the PCR was positive for each dilution from six total experiments is indicated. The markers for 933 and 951 bp correspond to der(2) and der(5) junctions, respectively, without end modification. The larger and smaller fragments seen in some of the lanes likely correspond to junctions with large insertions or deletions. (F) Detection of the NPM1–ALK fusion protein in Jurkat cells after TALNPM and TALALK coexpression and in the ALCL cell line SUPM2. The signal from 1 μg of cell extract from SUPM2 cells was compared with 40 μg from Jurkat cells. (G) Expanding pools of RPE-1 cells treated with TALENs. The PCR product corresponding to der(5) was detected when cells were split 1 to 10 every 3 d. The number of passages at each time point is indicated below the gel.











