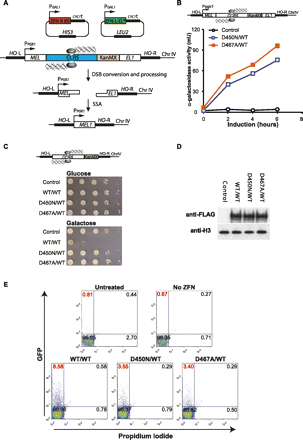
Targeted nicks stimulate homologous repeat deletion. (A) Illustration of the MEL1 reporter assay. A MEL1 reporter yeast strain, carrying the CCR5 ZFN target site introduced between two overlaping fragments of the MEL1 gene (MEL and EL1, respectively), was transformed with ZFN expression plasmids for ZFN-L-EL ([*] catalytically inactive FokI domain) and ZFN-R-KK, whose expression are under the control of galactose-inducible promoter (PGAL). ZFN expression was induced by the addition of galactose to the growth media and a DNA break or nick generated (depending on the constructs used). The repaired MEL1 gene directs the expression of α-galactosidase that can then be measured to determine the relative efficiencies of DNA break- or nick-induced repair. (CYC1t) Transcriptional terminator of CYC1 (cytochrome c1) gene, (HIS3) the gene encoding yeast imidazoleglycerol-phosphate dehydratase required for histidine biosynthesis, (LEU2) the gene encoding yeast beta-isopropylmalate dehydrogenase (IMDH) required for leucine biosynthesis; HIS3 and LEU2 are used for selection of transformants, (PPGK1) promoter of the phosphoglycerate kinase 1 (PGK1) gene, (KanMX) dominant resistance module for selection of S. cerevisiae transformants against geneticin (G418) (Voth et al. 2001). (B) Induction of a targeted nick in yeast stimulates homologous repeat deletion and restoration of the reporter gene (MEL1). ZFN expression was induced for 2 to 6 h, and reporter activity was determined as previously described (Doyon et al. 2008). (C) Evaluating direct double-strand DNA cleavage activity of the ZFNickases in yeast. A haploid yeast strain harboring the CCR5 ZFN target site integrated into the HO locus was transformed with the indicated galactose-inducible ZFNickase expression plasmids (D450N/WT, D467A/WT), DSB-inducing ZFN vectors (WT/WT), or a no ZFN control (Control). Tenfold serial dilutions of each cell line were plated on minimal media containing either glucose (ZFN expression off) or galactose (ZFN expression on) and incubated for 3 d. Direct DSB-induction results in a severe reduction in yeast cell viability (compare WT/WT+Glucose with WT/WT+galactose). No loss of viability is observed with the nickases. (D) Western blot to assess the expression levels of the various ZFN variants in panel C. Aliquots of the ZFN-induced cultures were harvested after 6 h of galactose induction. Cell lysates were run on SDS-PAGE, transferred, and blotted with either an anti-FLAG monoclonal antibody (Sigma) to detect FLAG-tagged ZFN or anti-H3 monoclonal antibody (Millipore) as loading control. No significant differences in ZFN expression were observed. (E) Induction of a targeted nick in mammalian cells stimulates homologous repeat deletion mediated restoration of reporter gene expression. K562 cells were either untreated or transfected with a GFP reporter DNA plasmid (Supplemental Fig. S1) in the absence (No ZFN) or presence of the indicated ZFN expression plasmids. Cells were collected 3 d post-transfection and subjected to flow cytometry analysis after a 5-min incubation with propidium iodide (PI) to stain nonviable cells (PI+). GFP+PI− cells (gate at top left) represent the number of viable cells which have restored marker gene expression. One representative example from three independent experiments is shown.











