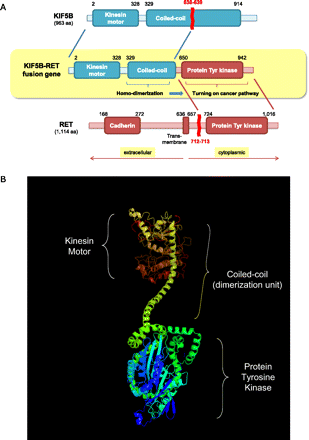
Molecular characteristics of KIF5B-RET fusion kinase. (A) Functional domains of KIF5B-RET fusion kinase. The fusion kinase consists of 638 N-terminal residues of KIF5B and 402 C-terminal residues of RET kinase. As a result, the fusion protein consists of a protein kinase domain together with a coiled-coil domain. The coiled-coil domain induces dimerization of the fusion kinase, which activates the oncogenic protein tyrosine kinase domain by autophosphorylation. (B) The three-dimensional structure of the KIF5B-RET chimeric oncogene, as predicted by the PHYRE2 algorithm (Kelley and Sternberg 2009). The N- and C-terminal of the fusion protein are colored in red and blue, respectively.











