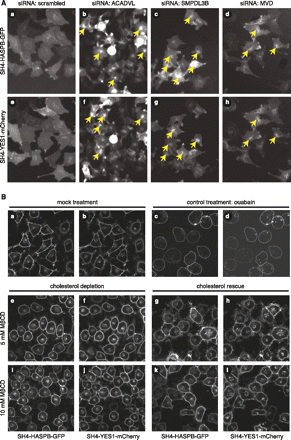
Interference with cellular lipid metabolism by RNAi-mediated down-regulation of metabolic enzymes or pharmacological cholesterol depletion causes intracellular retention of SH4 proteins. (A) HeLa cells expressing SH4-HASPB-GFP and SH4-YES1-mCherry were reverse-transfected with a scrambled siRNA (a,e) and siRNAs targeted against acyl-coenzyme A-dehydrogenase, very long chain (ACADVL) (b,f), sphingomyelin phosphodiesterase, acid-like, 3B (SMPDL3B) (c,g), and mevalonate (diphospho) decarboxylase (MVD) (d,h) and subjected to live-cell fluorescence microscopy. Enlarged image details from original data from the validation screen are shown. (B) HeLa cells expressing SH4-HASPB-GFP and SH4-YES1-mCherry were treated with methyl-β-cyclodextrin (MβCD) to deplete cellular cholesterol and analyzed by live-cell confocal microscopy to determine the subcellular localization of SH4 fusion proteins. Subsequently, the same cells were supplemented with cholesterol and analyzed by live-cell confocal microscopy again. Control cells were treated with 50 mM ouabain and analyzed by live-cell confocal microscopy.











