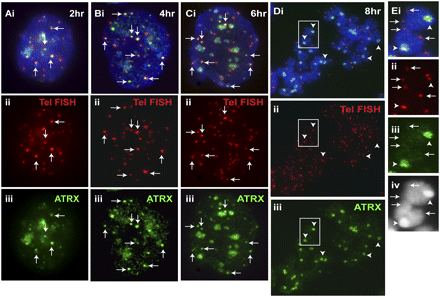
Timing of telomeric loading of ATRX. We previously showed that MYC-H3.3 was loaded at the telomeres in interphase ES129.1 cells during S phase (Wong et al. 2009). Here, ES129.1 cells were similarly synchronized using a thymidine-induced G1/S-block protocol (for FACS analysis, see Supplemental Fig. S3). (A–C) ATRX (iii, green) was present at the telomeres in cells from 2–6 h post-release from G1/S block, as indicated by colocalization with telomere FISH signals (ii, red). (D) By mitosis (8 h post-release), ATRX (iii, green) completely delocalized from the telomeres (ii, red; indicated by telomere FISH signals) but remained at pericentric heterochromatin (arrowheads). (E) Enlarged images of the boxed areas shown in D, showing no ATRX signals at the telomeres (arrows) but strong localization of ATRX (iii, green) at the pericentric regions (arrowheads); note that the telocentric nature of the mouse chromosomes meant an inevitable display of the observed colocalization of telomeric FISH signals with the centromeres at this resolution. At 10 h post-release, the cells re-entered G1 phase of the cell cycle (data not shown).











