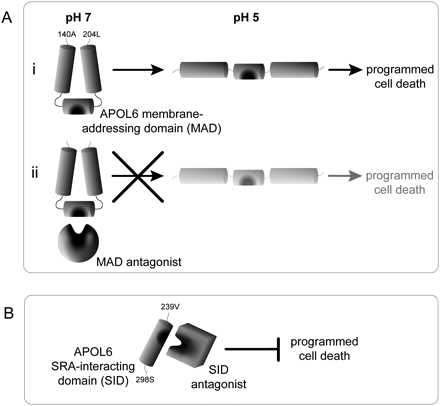
Cartoon model for how MAD and SID antagonists use distinct mechanisms to cause or prevent APOL6-mediated PCD of host cells. (A, i) In the absence of pathogen antagonists, we predict that the APOL6 MAD functions like the APOL1 MAD during trypanosome infections. That is, at pH 7 in an endosome, the MAD hinge is in closed conformation with salt bridges between adjacent alpha helices. At pH 5 in an endolysosome, the hinge opens and allows APOL1 to dissociate from HDL particles to form a pore in the lysosomal membrane, killing the trypanosome. An important difference between APOL1 and APOL6 is that secreted APOL1 kills trypanosomes, whereas APOL6 is not secreted and induces apoptosis of host cells. (ii) We predict that pathogen antagonists manipulate APOL6 function by interacting at the base of the MAD hinge—a region under strong positive selection (shown in dark gray). By recognizing this critical region, antagonists may be able to manipulate APOL6 in different ways. One possibility is that antagonists promote infections by preventing APOL6-mediated apoptosis. In this case, antagonists interact with the MAD hinge to keep it from opening (shown in figure), prolonging infection of the cell. Alternately, antagonists may further infections by prematurely causing apoptosis. In this case, antagonists could interact with the MAD base to open it, initiating apoptosis prematurely (not shown in figure). (B) The trypanosome antagonist SRA binds APOL1 to prevent APOL1-mediated trypanosome lysis during infections. The same region of APOL1 that SRA binds, the SID, is under positive selection in APOL6 (shown in dark gray) and other APOL genes. Hence, we predict that additional SID antagonists, like SRA but distinct in mechanism from MAD antagonists, interact with the SID to block APOL-mediated cell death of host cells during infections.











