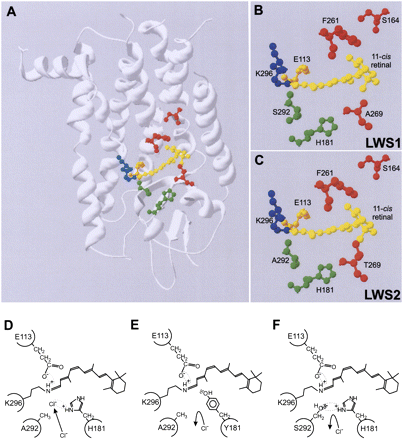
(A) Structural model of elephant shark LWS visual pigments showing the relative position of the five key LWS tuning sites (164, 181, 261, 269, and 292) within the retinal-binding pocket containing the retinylidene chromophore (yellow), the Lys296 chromophore attachment site via a Schiff base (blue), and the Glu113 counterion (orange). LWS tuning sites are color coded whether they are situated proximally to (green) or distally (red) from the Schiff base. Enlarged view showing the key residues of (B) LWS1 and (C) LWS2 pigments surrounding the retinylidene chromophore. The model was created using Swiss Model (Guex and Peitsch 1997) and is based on the crystal structure of bovine rhodopsin (Palczewski et al. 2000). A schematic representation of the proposed model for the interaction between residues 181 and 292 in close proximity to the Schiff base of the chromophore, showing (D) a chloride ion acting as a counterion to the positive charge present on His181, (E) the loss of binding and repulsion of a chloride ion due to the net negative polar charge of the Tyr residue at position 181, and (F) the net negative polar charge of the hydroxyl side chain of Ser292 repelling the negatively charged chloride ion and acting as a counterion to the positive charge present on His181. In all cases, the Glu113 counterion and Schiff base linking Lys296 and the retinylidene chromophore are shown. Putative electrostatic attractions between amino acid side chains and ions are highlighted by a dotted ellipse.











