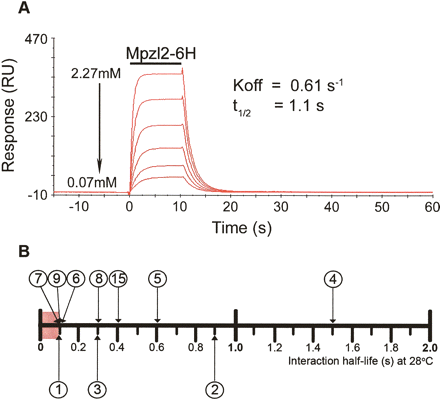
Validation and quantification of interactions by surface plasmon resonance. (A) Reference-subtracted surface plasmon resonance traces of soluble Mpzl2-Cd4-6H protein interacting with surface-immobilized Mpzl3-Cd4-bio. The binding curves of six twofold serial dilutions (as shown) of the purified protein were injected over 1016RU of immobilized Mpzl3 as indicated by the bar. The wash-out phase of the binding was fitted to a 1:1 dissociation model, and the off-rate constant (koff) was determined and a half-life calculated (see Methods). (B) Relative monomeric half-lives of interactions quantified by SPR as measured at 28°C. Interactions are numbered as in Fig. 2. Heterophilic interactions are placed above the scale, and homophilic interactions below. The red box represents the limits of SPR sensitivity, suggesting that several interactions have half-lives ≤ 0.1 sec.











