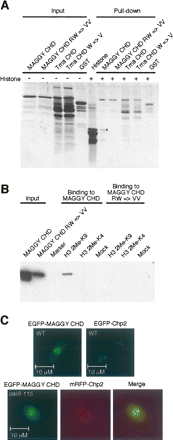
The MAGGY CHD recognizes histone H3 methyl-K9. (A) MAGGY CHD interacts with histone H3. GST-MAGGY CHD and GST-Tma CHD fusion proteins were mixed with a calf thymus histone extract and then pulled down using glutathione agarose beads. Both the input and pull-down reactions were separated by SDS-PAGE and visualized by SYPRO Ruby protein gel stain. H3, as indicated by the asterisk, was enriched in the pull-down with the MAGGY CHD. No interaction was observed with the MAGGY CHD RW mutation or with wild-type or mutant variants of the Tma CHD. (B) The MAGGY CHD specifically interacts with H3 dimethyl-K9. Biotin-labeled histone peptides were incubated with His6-tagged MAGGY CHD, and then pulled-down with streptavidin agarose beads. Pull-down reactions were separated by SDS-PAGE and transferred to nylon membranes. The presence of the CHD was detected using an anti-His6 antibody. The MAGGY CHD specifically interacts with histone H3 dimethyl-K9, and not with H3 dimethyl-K4. Mutations in conserved residues of the CHD abrogate the interaction. (C) MAGGY CHD localizes to sites of H3 methyl-K9 in S. pombe. The MAGGY CHD localizes to punctate sites within the nucleus as does Chp2, a CHD protein found at sites of centromeric heterochromatin in S. pombe (Sadaie et al. 2004) (top). Colocalization of the MAGGY CHD and Chp2 is observed in a swi6-115 strain, which lacks the S. pombe HP1 homolog (Nakagawa et al. 2002). The swi6-115 strain should allow expression of genes in centromeric heterochromatin and was used in transposition assays that require expression of a marker gene carried by Tf1 (see Fig. 4).











