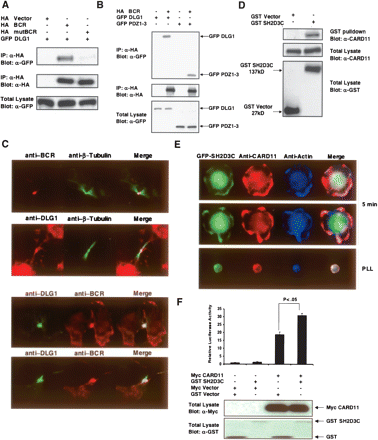
Experimentally verified novel PDZ complexes. (A) DLG1 binds the break point cluster region (BCR) protein. 293T cells were co-transfected with wild-type HA-tagged BCR or PDZ-binding motif mutant (mutBCR) and GFP-tagged DLG1. Anti-HA antibody immunoprecipitates were resolved by SDS-PAGE and immunoblotted for GFP-DLG1. (Upper panel) Proteins retained on Ha beads were probed with anti-GFP antibody; (middle panel) cell extracts probed with anti-Ha antibody; (lower panel) cell extracts probed with anti-GFP. (B) Co-immunoprecipitation demonstrating that DLG1 PDZ domains are sufficient for binding to BCR. Co-transfection of HA epitope-tagged BCR along with full-length wild-type GFP-DLG1 or the isolated DLG1 PDZ domains 1–3. (Upper panel) Proteins retained on anti-HA beads probed with anti-GFP antibody; (middle panel) cell extracts probed with anti-HA antibody; (bottom panel) cell extracts probed with anti-GFP antibody. (C) Staining of endogenous BCR-DLG1 shows their co-localization at the midbody in U20S cells during cytokinesis. (D) SH2D3C associates with CARD11. Interaction between GST-tagged SH2D3C with endogenous CARD11 in Jurkat cells. Expression constructs encoding GST vector and GST-SH2D3C were electroporated into Jurkat cells, and 24 h post-transfection lysates were prepared and subjected to GST pull-down. The immunoprecipitates (IP) and total lysates (TL) were resolved by SDS-PAGE and immunoblotted with anti-CARD11 and anti-GST. (E) GFP-SH2D3C and CARD11 co-localize upon cell spreading in response to T-cell stimulation. Jurkat cells expressing GFP-SH2D3C (green) were activated to induce spreading for 5 min at 37°C on glass slides coated with anti-CD3 and CD28 antibodies (two examples shown in two upper panels). As a control, cells were also stained on poly-lysine-coated slides (PLL) lacking anti-CD3 and anti-CD28. After fixation, cells were stained with rabbit anti-CARD11 followed by anti-rabbit IgG conjugated with Alexa-Flour 568 (red) and phalloidin conjugated with Alexa-Flour 350 (blue–actin). These results represent one of three independent experiments, which gave similar results. (F) SH2D3C activates the CARD11-mediated IL-2 activation. Jurkat E6 cells (5 × 106) were electroporated with GST-Vector (8 μg) or GST-SH2D3C (8 μg), Myc-vector (5 μg), or Myc-CARD11 (5 μg), along with two reporter plasmids (7 μg of IL-2-luc and 0.2 ng of renilla-luc). After 24 h of electroporation, cells were harvested, lysed, and examined for luciferase activity, showing a significant increase in IL-2 promoter activation upon co-transfection of both genes (student’s t-test P < 0.05). (Lower panel) Expression levels of the transfected proteins.











