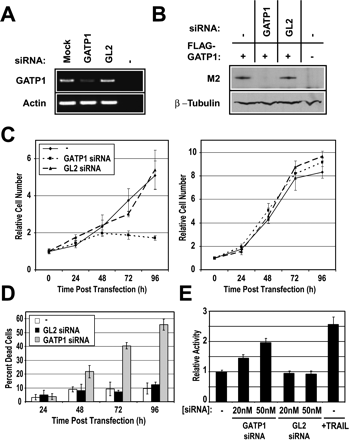
Gatp1 is required for cell growth and viability under reduced serum conditions. Target mRNA and protein knockdown by Gatp1 siRNA was confirmed by (A) semiquantitative RT-PCR and (B) immunoblot analysis. RNAs harvested from NIH3T3 cells transfected with the indicated siRNA were analyzed using actin and Gatp1 gene-specific primers. Lysates prepared from NIH3T3 cells transfected with Gatp1 or control (GL2) siRNAs, along with a cDNA encoding Gatp1-Flag fusion protein, as indicated, were immunoblotted with M2 anti-Flag (B, top panel) and β-tubulin antibodies (B, bottom panel). (C) Gatp1 expression is required for proliferation under low-serum conditions. Approximately 1000 NIH3T3 cells seeded into 384-well plates at low (left panel) and normal (right panel) serum were mock-transfected (–•–) or transfected with Gatp1 (–▪–) or GL2 (–▴–) siRNAs. Cell numbers at each condition were evaluated by imaging over the indicated time period. Cells were visualized by staining with Hoechst 33342. (D) Abrogation of Gatp1 expression at low-serum conditions increases cell death. Images from C were analyzed to evaluate the effects of Gatp1 and GL2 (control) siRNAs on cell cycle distribution (Wan et al. 2004). Histograms plotting the number of events versus total nuclear fluorescence for sample wells were used to define boundaries for the sub-G1 population. Results are presented as percentages of total cells in each well. Samples were prepared in quadruplicate for each condition, and replicate wells were averaged. (E) siRNA-mediated depletion of Gatp1 activates primary effector caspase-3 and caspase-7. NIH3T3 cells transfected with the indicated siRNA at 1% serum were analyzed using the Apo-ONE Homogeneous Caspase-3/7 Assay kit. Untransfected cells treated with recombinant human TRAIL were analyzed as positive control.











