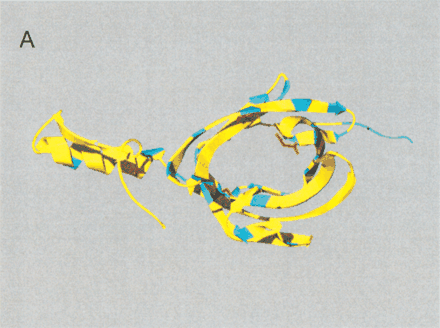
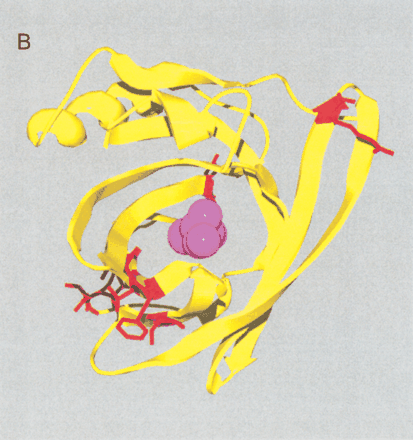
(A) Site-specific KA/KS analysis of OBPs. ω+ codons with a posterior probability >0.90 by one codeml model, and >0.5 by at least one other model, were mapped to the crystal structure of cow OBP (PDB: 1OBP; Bianchet et al. 1996) and colored blue. Three ω+ site side-chains, shown in ball-and-stick format and colored red, are predicted to project into the interior of the OBP β-barrel. Two of these side-chains (1OBP:T38 and V69) are within 5 Å of the ligand that was cocrystalized in the 1OBP X-ray structure, whereas the third side-chain (1OBP:E84) is more distant from the ligand (∼8 Å), and closer to the cavity edge. (B) Positively selected sites shared by both MUP/α2u-globulins and OBPs. A multiple alignment of MUP/α2u-globulins and OBPs was used to identify nine ω+ codons shared by both families. These sites are spatially clustered and therefore represent a hypervariable region likely to be important for functional diversity. The nine ω+ codons were mapped to the crystal structure of mouse MUPs (PDB 1MUP) and are colored red. 2-(Sec-butyl) thiazoline is shown in purple.











