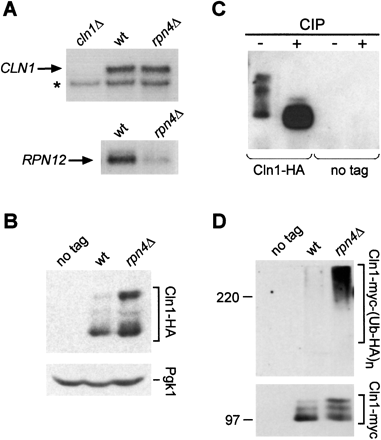
Stabilization of Cln1 protein in rpn4Δ mutants. (A) Northern blot analysis of total RNA from wild-type and rpn4Δ strains grown on SLAD plates, and a cln1Δ strain. The blot was probed consecutively with probes for CLN1 and RPN12. The asterisk in the CLN1 blot indicates a cross-hybridizing band that also serves as a loading control. (B) Western blot analysis of Cln1 protein in diploid wild-type and rpn4Δ strains carrying HA-tagged CLN1, and a no-tag wild-type control strain. Protein extracts were prepared from cells grown for 10 h on SLAD agar plates. Pgk1 protein levels served as a loading control. (C) Cln1-HA protein was immunoprecipitated from an rpn4Δ strain. Aliquots of the immunoprecipitate were incubated with calf-intestine phosphatase (CIP), or without CIP, and analyzed by Western blotting. (D) myc-tagged Cln1 protein was immunoprecipitated in diploid wild-type and rpn4Δ strains, and a no-tag control strain. All strains had a multicopy plasmid expressing HA-tagged ubiquitin. Immunoprecipitates were analyzed by gel electrophoresis and immunoblotting with anti-HA antibody to detect ubiquitin conjugates. The blot membrane was stripped and reprobed with anti-myc antibodies to detect the immunoprecipitated Cln1.











