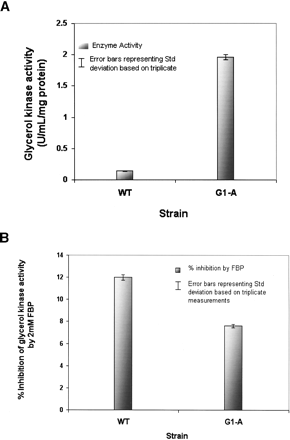
Enzyme kinetics of the glycerol kinase variant (G692A, Gly232Asp) of E. coli G1-A in comparison to the wild-type enzyme (wt). Enzyme activities were determined as a decrease in absorbance at 340 nm monitoring ADP release by coupling pyruvate kinase and lactate dehydrogenase activity as detailed in Methods. (A) A 12-fold increase in enzyme activity in the recombinant mutant clone as opposed to the wild type. (B) Enzyme inhibition by an allosteric inhibitor Fructose-1,6-bisphosphate (FBP) was determined by addition of FBP to a final concentration of 2 mM. Inhibitory effects decreased by ∼33% in the enzyme variant when compared to the wild-type enzyme.











