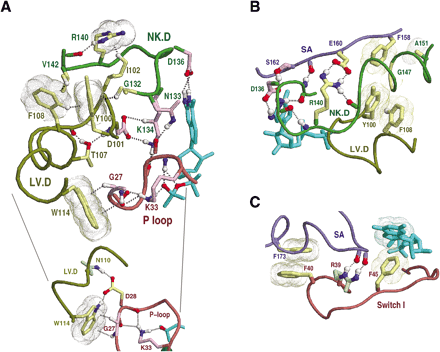
Sec4p as a structural prototype of FY-pivot GTPases. The structure of Sec4p is shown in complex with GDP (pdb code: 1G16). The corresponding hierarchical alignment is given in Figure 3. Hydrogen bonds are depicted as dotted lines, and aromatic-aromatic and van der Waals interactions as dot clouds. Dotted lines into clouds depict CH-π or NH-π interactions (Weiss et al. 2001). Color scheme: GDP (cyan); main-chain traces and residue designations (colored by regions as indicated in Fig. 2 E); residue side-chains and canonical glycine main-chains (color scheme of Fig. 2 D); oxygen, nitrogen, and hydrogen atoms establishing hydrogen bonds (red, blue, and white, respectively); hydrogen bonding carbons (colored as their corresponding side-chains). Figures were generated using RasMol (Sayle and Milner-White 1995). (A) Canonical interactions between the LV.D and NK.D regions. These include a perpendicular aromatic-aromatic interaction (F108-Y100), four CH-π interactions (F108-Y100, G132-Y100, V142-F108, and I102-R140), and main-chain hydrogen bonds to two side-chains (T107 and R140). Y100 is the FY-pivot residue. The inset highlights interactions between the LV.D and P-loop regions. (B) Canonical interactions within and between the NK.D and SA regions. These include packing of a phenylalanine or tyrosine (F158) against two small residues (G147 and A151) in the helix following the NK.D loop, a salt bridge (R140 and E160), and several main-chain hydrogen bonds. Residues within the LV.D region are shown for comparison with A. (C) Canonical interactions between the SA and Switch I regions. Note that, unlike Ran (Figs. 7 D,8), there are no major structural differences between the GDP and GTP-forms of Sec4p in these regions. Also shown is a previously noted (Hall 2000) canonical aromatic-aromatic interaction between a phenylalanine (F45) and bound guanine. Residues homologous to R39 (mainly arginine, glutamine, or serine), though inconsistently conserved at the sequence level, conserve hydrogen bonding interactions with the SA region.











