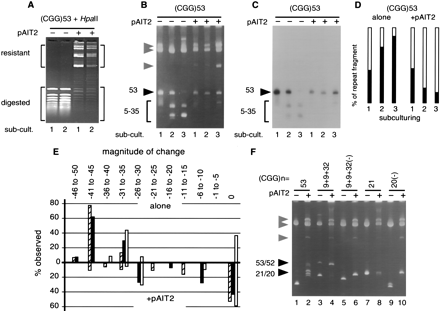
Effect of methylation on the genetic stability of fragile X (FRAXA) (CGG)n repeats. (A) The pFXA53 plasmid containing (CGG)53 (Table 1) was propagated in Escherichia coli in the absence or presence of the SssI methylase-expressing plasmid pAIT2. The methylation status of the DNAs was monitored by methyl-sensitiveHpaII restriction digestion. Shown is the analysis of the first and second subculturings (each representing 25 generations) of pFXA53. (B) After three subculturings, an aliquot of cells was harvested and plasmids were isolated, restriction digested, and analyzed by 4% polyacrylamide gel electrophoresis (PAGE) and ethidium bromide staining. To test for repeat length changes, DNAs were digested with KpnI and SacI, which liberates the (CGG)n-containing fragments from the pFXA clones, which are resolvable from pAIT2 and vector bands. The starting (CGG)n lengths are indicated by filled arrowheads; all other faster migrating DNAs are products of (CGG)n deletions indicated by vector bands, indicated by shaded arrowheads. This gel is representative of five or more independent experiments. (C) To confirm that products were caused by changes in repeat numbers, the gel shown in panel A was electrotransferred to nylon membrane and hybridized to32P-labeled (CGG)10 oligonucleotides and exposed for autoradiography. (D) The effect of methylation (±pAIT2) on repeat stability was determined by densitometric analysis of multiple experiments as described (Kang et al. 1995). The open area represents the percentage of material with the starting repeat length, whereas the filled area represents the percentage of deletion products. (E) The magnitude of repeat loss was determined by streaking on plates cells from each subculturing of a test plasmid in the absence or presence of methylation (±pAIT2), isolation of ≥20 single colonies, and analysis of their DNA. The analysis of pFXA53 is shown. Deletion sizes for the first, second, and third subculturings are shown by hollow, filled, and cross-hatched bars, respectively. (F) FRAXA plasmids containing the indicated lengths, purities, and orientations of (CGG)n tracts (Table1) were propagated through three subculturings in E. coli in the absence or presence of methylation (± pAIT2). After each subculturing, cells were harvested and (CGG)n repeat length changes analyzed as above. The trend through the three subculturings is reflected by the final subculture; hence only the third subculturing is shown for each plasmid. This gel is representative of five or more independent experiments. Products are labeled as in panel B.











