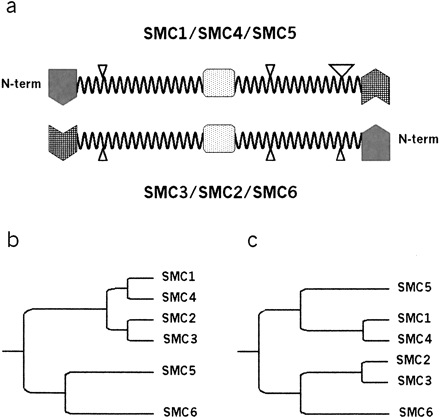
The conserved disruptions to the predicted coiled-coil arm domains of eukaryotic SMC heterodimers abolish SMC structural symmetry and indicate a common ancestral complex for all eukaryotic SMC heterodimers. (a) Schematic representation of the domain structure of cohesin, condensin, and SMC5-SMC6 SMC heterodimer. The globular N-terminal (shaded) and C-terminal (hatched) domains are separated by two helical coiled-coil arms and a central hinge domain (dots). Coiled-coil disruptions to the arm domains (loops) are indicated by triangles with size that is proportional to the length of the loop. Because of the conserved domain architecture of SMC proteins and the antiparallel association of the heterodimers, SMC complexes lack secondary structure orientation and can be freely rotated around the hinge domain and about a lateral axis along the arm lengths. However, with an appreciation of the observed disruptions to the coiled-coil domains as structural features of eukaryotic SMCs, the heterodimers gain a structural directionality that may translate into a functional orientation of the complexes. Not to scale. (b) A dendrogram of eukaryotic SMC evolution derived from the primary sequences of the cohesin, condensin, and SMC5-SMC6 heterodimers as presented by Cobbe and Heck (2000) and Jones and Sgouros (2001). It differs from a phylogeny that incorporates the structural similarities between all eukaryotic heterodimeric SMC complexes (c), which have a conserved distribution of disruptions to their coiled-coil domains. Arm distances are only illustrative of evolutionary distances of SMC subfamilies.











