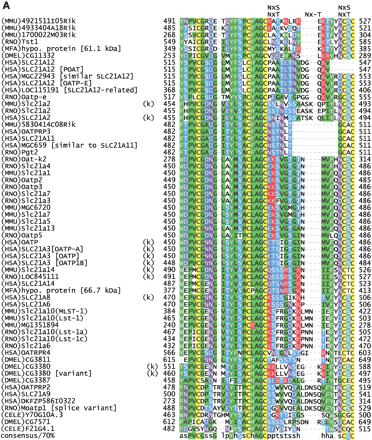
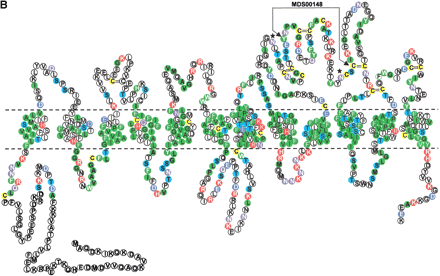
(A) Multiple sequence alignment of extracellular domain containing the OATP family motif MDS00148. The proteins are designated by the species abbreviation and the name. The positions of motif MDS00148 are designated by “+”. Potential N-glycosylation sites N-x-[ST] are indicated. A multiple sequence alignment of MDS00148 representatives covering the entire sequence length and a phylogenetic tree are shown in Suppl. Fig. 3A and B. The SWISS-PROT/TrEMBL accessions of the sequences are as follows: (MMU)4921511I05Rik, Q9D5W6; (MMU)4933404A18Rik, Q9D4B7; (MMU)1700022M03Rik, Q9DA23; (RNO)Tst1,AAK63015; (MFA)hypo. protein [61.1 kD], BAB69708; (DMEL)CG11332, Q9I7P1; (HSA)SLC21A12, Q9H4T8; (HSA)SLC21A12 [POATP], Q9UI35; (HSA)MGC22943 [similar to SLC21A12], AAH15727; (HSA)SLC21A12 [OATP-E], Q9UIG7; (HSA)LOC115191 [SLC21A12-related], Q9H8P2; (RNO)Oatp-e, Q99N01; (MMU)Slc21a2, Q9EPT5; (RNO)Slc21a2, Q00910; (HSA)SLC21A2, Q92959; (MMU)5830414C08Rik, Q9JKV0; (HSA)OATPRP3, Q9GZV2; (HSA)SLC21A11, Q9UIG8; (HSA)MGC659 [similar to SLC21A11], Q9BW73, (RNO)Pgt2, Q99N02; (RNO)Oat-k2, Q9WTM0; (RNO)Slc21a4, P70502; (MMU)Slc21a1, Q9QXZ6; (RNO)Oatp2, O35913; (RNO)Oatp3, O88397; (RNO)Slc21a7, Q9EQR8; (RNO)Slc21a3, P46720; (MMU)MGC6720, AAH13594; (MMU)Slc21a7, AAK39416; (MMU)Slc21a5, Q9EP96; (MMU)Slc21a13, Q99J94; (RNO)Oatp5, Q9QYE2; (HSA)OATP, AAG30037; (HSA)SLC21A3 [OATP-A],CAB97006; (HSA)SLC21A3 [OATP], P46721; (HSA)SLC21A3 [OATP1B], Q9UL38; (MMU)Slc21a14, Q9ERB5; (RNO)LOC845111, Q9EPZ7, (HSA)SLC21A14, Q9NYB5; (MFA)hypo. protein [66.7 kD], Q9GMU6; (HSA)SLC21A8, Q9NPD5; (HSA)SLC21A6, Q9Y6L6; (MMU)Slc21a10(MLST-1), Q9JJJ1; (MMU)Slc21a10(Lst-1), Q9JJL3; (MMU)MG1351894, Q9JI79; (RNO)Slc21a10(Lst-1a), Q9JHF6; (RNO)Slc21a10(Lst-1c), Q9JIM2; (RNO)Slc21a6, Q9QZX8; (HSA)OATRPR4, Q9H2Y9; (DMEL)CG3811, Q9VLB3; (DMEL)CG3380, Q9W269; (DMEL)CG3380 [variant], AAK77236; (DMEL)CG3387, Q9W271; (HSA)OATPRP2, Q9H2Z0; (HSA)SLC21A9, O94956; (HSA)DKFZP586I0322, Q9UFU1; (RNO)moatp1 [splice variant], Q9JHI3; (CELE)Y70G10A.3, Q9XWC5; (DMEL)CG7571, Q9VVH9; and (CELE)F21G4.1, Q93550. Motif sequences that contain an hmmpfam-predicted kazal-type serine protease inhibitor domain are labeled with a “k”. (B) Proposed topology of an organic anion transporter. The primary structure is shown for 4921511I05Rik, assuming 12 transmembrane helices. The intra- and extracellular domains are separated by horizontal dashed lines, which symbolize the cell membrane. The motif region MDS00148 is indicated by arrows. Potential extracellular N-glycosylation sites are symbolized by asterisks. Potential disulfide bonds of Cys residues are shown as double lines.











