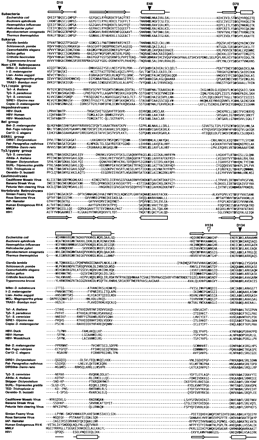
Alignment of the Ribonuclease HI (RNH) domains. Representative RNH domains from Eubacteria, Eukarya, non–LTR retrotransposons and each of the seven lineages of LTR retrotransposons were aligned usingCLUSTALX and PSI-BLAST. Highlighted in bold are the residues believed important for the catalytic mechanism of RNH, including the four carboxylate (dark arrows) and the single histidine residue (white arrow) that are numbered according to their position in the Escherichia coli RNH domain. Also overlaid are the secondary structures of E. coli and HIV-1 RNH domains (above and below the alignment, respectively). Note the missing histidine residue in all lineages of the LTR retrotransposons except the vertebrate retroviruses.











