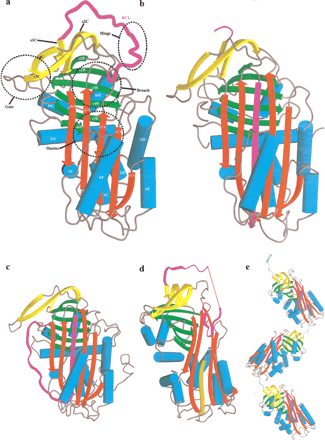
(A) The structure of native α1-antitrypsin. (B) Cleaved α1-antitrypsin. (C) Latent antithrombin. (D) δ-Antichymotrypsin. Part of the F-helix is unwound and inserted into the bottom of the A β-sheet (orange). (E) Polymer of cleaved antitrypsin. Residues P5–P4′ in the RCL, part of which (P5–P1) are making the β-strand linkage, are shown in light green. In all parts of Figure 1, the A β-sheet is in red, the B β-sheet in green, the C β-sheet in yellow, and the reactive center loop (RCL) in magenta. The helices are represented by cylinders colored cyan. Elements of secondary structure are labeled as follows: (hA, hB, etc.) A-helix, B-helix, etc.; (s1A, s2A, etc.) strand 1 of the A β-sheet, strand 2 of the A β-sheet, etc. The important breach, shutter, gate, and hinge regions are indicated by broken circles.











